News & Views |
Featured
-
-
News & Views |
 p21 shapes cancer evolution
p21 shapes cancer evolution
Although known to induce cellular senescence, an important tumour suppressor mechanism, mutation of CDKN1A — the gene encoding p21 (also known as WAF1 or CIP1) — is rare in human cancers. Now, a study reports a previously unappreciated oncogenic effect of p21 overexpression that shapes cancer genome evolution through induction of replication stress.
- Vasily S. Romanov
- & K. Lenhard Rudolph
-
Article |
 Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer
Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer
Walerych et al. show that p53 missense mutants upregulate the proteasome and increase breast cancer cell resistance to proteasome inhibitors. Combined inhibition of p53 mutants and the proteasome leads to increased therapeutic efficacy.
- Dawid Walerych
- , Kamil Lisek
- & Giannino Del Sal
-
Article |
 Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing
Chronic p53-independent p21 expression causes genomic instability by deregulating replication licensing
Galanos and colleagues observe p21 accumulation in proliferating cancer cells, and show that in cultured p53-null cells, p21 can cause genomic instability by perturbing replication licensing through inhibition of the CRL4-CDT ubiquitin ligase.
- Panagiotis Galanos
- , Konstantinos Vougas
- & Vassilis G. Gorgoulis
-
Article |
 Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells
Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells
Lee et al. report that aberrantly folded cytosolic proteins are recruited to the ER by the USP19 deubiquitylase, and are then encapsulated in late endosomes that fuse with the plasma membrane, leading to their secretion.
- Jin-Gu Lee
- , Shokichi Takahama
- & Yihong Ye
-
Review Article |
 The increasing complexity of the ubiquitin code
The increasing complexity of the ubiquitin code
Yau and Rape discuss recent advances in our understanding of the many variations in ubiquitin chain topology and how these mediate ubiquitin-dependent signalling in the cell.
- Richard Yau
- & Michael Rape
-
Letter |
 Godzilla-dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs
Godzilla-dependent transcytosis promotes Wingless signalling in Drosophila wing imaginal discs
Vincent and colleagues show in Drosophila wing imaginal discs that the signalling molecule Wingless is synthesized and secreted at the apical surface, and is re-internalized to be transcytosed basally, where its signalling occurs.
- Yasuo Yamazaki
- , Lucy Palmer
- & Jean-Paul Vincent
-
News & Views |
 Ramping up degradation for proliferation
Ramping up degradation for proliferation
The control of proteasome-mediated protein degradation is thought to occur mainly at the level of polyubiquitylation of the substrate. However, the proteasome can also be regulated directly, as now demonstrated by a study in which DYRK2-mediated phosphorylation of the 19S subunit Rpt3 is found to increase proteasome activity.
- Jon M. Huibregtse
- & Andreas Matouschek
-
Article |
 Site-specific proteasome phosphorylation controls cell proliferation and tumorigenesis
Site-specific proteasome phosphorylation controls cell proliferation and tumorigenesis
Dixon and colleagues and Guo and colleagues find that phosphorylation of the 19S proteasome subunit Rpt3 by DYRK2 increases proteasome activity and promotes cell proliferation, whereas loss of Rpt3 phosphorylation inhibits tumour formation in mice.
- Xing Guo
- , Xiaorong Wang
- & Jack E. Dixon
-
Article |
 IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation
IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation
Through a proteomics approach, Qi and colleagues and Long and colleagues identify the sensor of the unfolded protein response IRE1α as an endogenous substrate of the E3 ubiquitin ligase involved in ER-associated degradation, Hrd1.
- Shengyi Sun
- , Guojun Shi
- & Ling Qi
-
Resource |
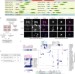 Systematic proteomics of the VCP–UBXD adaptor network identifies a role for UBXN10 in regulating ciliogenesis
Systematic proteomics of the VCP–UBXD adaptor network identifies a role for UBXN10 in regulating ciliogenesis
Through proteomics, Harper and colleagues identify proteins interacting with UBXD adaptors for the multifunctional AAA-ATPase VCP and reveal a role for UBXN10 in ciliogenesis.
- Malavika Raman
- , Mikhail Sergeev
- & J. Wade Harper
-
Article |
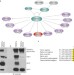 FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification
FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification
Aifantis and colleagues report that FBXW7α controls the heat-shock response pathway by targeting HSF1 for degradation. In melanoma FBXW7α deficiency leads to increased nuclear HSF1, and induction of a pro-invasive gene expression program.
- Nikos Kourtis
- , Rana S. Moubarak
- & Iannis Aifantis
-
Article |
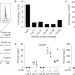 USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria
USP30 and parkin homeostatically regulate atypical ubiquitin chains on mitochondria
Cunningham et al. characterize the ubiquitin chain linkages regulated by the opposing activities of the E3 ligase parkin and the deubiquitylation enzyme USP30 on mitochondria.
- Christian N. Cunningham
- , Joshua M. Baughman
- & Jacob E. Corn
-
News & Views |
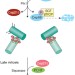 Solving the centriole disengagement puzzle
Solving the centriole disengagement puzzle
The microcephaly protein, Cep215, contributes to the engagement of duplicated centrioles in interphase. Now two distinct pools of Cep215 at centrosomes are identified, one bound to Cep68 and the other to pericentrin. Plk1-mediated degradation of Cep68 and separase-mediated cleavage of pericentrin release both pools of Cep215, thereby promoting centriole disengagement.
- Andrew M. Fry
-
Article |
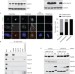 Degradation of Cep68 and PCNT cleavage mediate Cep215 removal from the PCM to allow centriole separation, disengagement and licensing
Degradation of Cep68 and PCNT cleavage mediate Cep215 removal from the PCM to allow centriole separation, disengagement and licensing
Pagano and colleagues find that Plk1 and the E3 ubiquitin ligase SCFβTrCP mediate degradation of the centrosome cohesion protein Cep68 and show this mediates removal of Cep215 from the PCM and subsequent centriole separation in late mitosis.
- Julia K. Pagan
- , Antonio Marzio
- & Michele Pagano
-
News & Views |
 Rsp5/Nedd4 clears cells of heat-damaged proteins
Rsp5/Nedd4 clears cells of heat-damaged proteins
Protein quality control systems protect cells from proteotoxicity caused by the accumulation of aberrantly folded polypeptides. The Rsp5 ubiquitin ligase (mammalian homologue Nedd4) is now identified as a major constituent of a clearance pathway that degrades misfolded cytosolic proteins after exposure to heat.
- Thomas Sommer
- , Annika Weber
- & Ernst Jarosch
-
Article |
 Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress
Rsp5/Nedd4 is the main ubiquitin ligase that targets cytosolic misfolded proteins following heat stress
Mayor and colleagues identify yeast Rsp5 (mammalian homologue Nedd4) as the main ubiquitin ligase responsible for the increased ubiquitylation following heat stress when Rsp5 targets cytosolic misfolded proteins for proteasome degradation.
- Nancy N. Fang
- , Gerard T. Chan
- & Thibault Mayor
-
Resource |
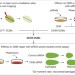 Systematic characterization of deubiquitylating enzymes for roles in maintaining genome integrity
Systematic characterization of deubiquitylating enzymes for roles in maintaining genome integrity
Systematic characterization of deubiquitylating enzymes in the DNA-damage response identifies UCHL5 as promoting DNA-end resection.
- Ryotaro Nishi
- , Paul Wijnhoven
- & Stephen P. Jackson
-
Article |
 ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1
ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1
Ma and colleagues show that when the EMT-associated transcription factor ZEB1 is stabilized by the ATM kinase, it interacts with the ubiquitin protease USP7 to counteract CHK1 degradation and promote DNA repair in breast cancer cells.
- Peijing Zhang
- , Yongkun Wei
- & Li Ma
-
-
Article |
 Der1 promotes movement of misfolded proteins through the endoplasmic reticulum membrane
Der1 promotes movement of misfolded proteins through the endoplasmic reticulum membrane
How misfolded proteins are extracted from the endoplasmic reticulum (ER) for degradation remains unclear. Sommer and colleagues demonstrate that following assembly into the HRD ligase complex, Der1 forms oligomers in the ER membrane and enables extraction of proteins from the ER lumen.
- Martin Mehnert
- , Thomas Sommer
- & Ernst Jarosch
-
Article |
 Deubiquitylation and stabilization of PTEN by USP13
Deubiquitylation and stabilization of PTEN by USP13
The stability of the PTEN tumour suppressor protein is regulated by polyubiquitylation. Ma and colleagues identify USP13 as an enzyme reversing polyubiquitylation of PTEN, leading to PTEN stabilization and tumour suppression.
- Jinsong Zhang
- , Peijing Zhang
- & Li Ma
-
News & Views |
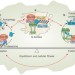 Q-bodies monitor the quinary state of the protein fold
Q-bodies monitor the quinary state of the protein fold
Cytoplasmic compartments containing misfolded proteins targeted for degradation, named Q-bodies, have been identified. Q-body formation is a dynamic process that actively manages the metastable state of the protein fold through small heat shock proteins and the Hsp70–Hsp90–Hsp110 proteostasis system to promote cellular fitness under both physiological and stress conditions.
- Daniela Martino Roth
- & William E. Balch
-
Article |
 The deubiquitylase USP33 discriminates between RALB functions in autophagy and innate immune response
The deubiquitylase USP33 discriminates between RALB functions in autophagy and innate immune response
The RAS-like GTPase RALB mediates cellular responses to nutrient availability or viral infection by engaging two distinct exocyst complex proteins: EXO84 to modulate autophagy and SEC5 to regulate innate immune signalling. Sablina and colleagues find that whereas ubiquitylation of RALB at K47 promotes its interaction with SEC5, the de-ubiquitylase USP33 switches RALB to the EXO84–beclin complex to promote autophagy during nutrient starvation.
- Michal Simicek
- , Sam Lievens
- & Anna A. Sablina
-
Article |
 Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress
Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress
Quality control of misfolded proteins is thought to involve proteasome-dependent degradation or, if this fails, sequestration into inclusion bodies. Frydman and colleagues reveal the existence of endoplasmic-reticulum-associated structures, termed Q-bodies, that concentrate misfolded proteins in a chaperone-dependent manner before degradation.
- Stéphanie Escusa-Toret
- , Willianne I. M. Vonk
- & Judith Frydman
-
Letter |
 Role of Cdc48/p97 as a SUMO-targeted segregase curbing Rad51–Rad52 interaction
Role of Cdc48/p97 as a SUMO-targeted segregase curbing Rad51–Rad52 interaction
The segregase Cdc48 (also called p97 or VCP) extracts ubiquitylated proteins from their environment. Jentsch and colleagues demonstrate that Cdc48 binds SUMOylated Rad52, and removes Rad52 and the recombinase Rad51 from DNA to restrict spontaneous recombination.
- Steven Bergink
- , Tim Ammon
- & Stefan Jentsch
-
Article |
 FBXL2- and PTPL1-mediated degradation of p110-free p85β regulatory subunit controls the PI(3)K signalling cascade
FBXL2- and PTPL1-mediated degradation of p110-free p85β regulatory subunit controls the PI(3)K signalling cascade
Phosphatidylinositol-3-OH kinase (PI(3)K) signalling regulates many cellular events such as cell growth and survival. Pagano and colleagues show that the E3 ligase SCF-FBXL2 promotes PI(3)K signalling and inhibits autophagy by targeting the free p85β subunit of the p110–p85 PI(3)K complex for degradation.
- Shafi Kuchay
- , Shanshan Duan
- & Michele Pagano
-
-
News & Views |
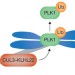 Cullin' PLK1 from kinetochores
Cullin' PLK1 from kinetochores
To ensure proper attachment of all chromosomes to the spindle, PLK1 has to associate with kinetochores during prometaphase and must be released from these sites before sister chromatid separation can begin. The monoubiquitylation of PLK1 by the ubiquitin ligase CUL3–KLHL22 is now identified as a critical step in promoting the release of PLK1 from kinetochores, pushing non-proteolytic ubiquitylation into the limelight of cell division research.
- Colleen A. McGourty
- & Michael Rape
-
Article |
 Ubiquitylation-dependent localization of PLK1 in mitosis
Ubiquitylation-dependent localization of PLK1 in mitosis
The dynamic localization of PLK1 to kinetochores is required for faithful chromosome segregation. Peter, Sumara and colleagues demonstrate that a KHL22-containing E3 ligase mediates degradation-independent removal of PLK1 from kinetochores to ensure satisfaction of the spindle assembly checkpoint and proper mitotic progression.
- Jochen Beck
- , Sarah Maerki
- & Izabela Sumara
-
Article |
 SCFFbxw5 mediates transient degradation of actin remodeller Eps8 to allow proper mitotic progression
SCFFbxw5 mediates transient degradation of actin remodeller Eps8 to allow proper mitotic progression
Cortical actin is implicated in cell shape regulation during mitosis. Melchior and colleagues reveal that SCFFbxw5-mediated ubiquitylation and degradation of the actin remodeller Eps8 is required for timely cell rounding and progression into metaphase, whereas the capping activity of Eps8 is needed for mitotic exit.
- Achim Werner
- , Andrea Disanza
- & Frauke Melchior
-
Article |
 SCFFbxo9 and CK2 direct the cellular response to growth factor withdrawal via Tel2/Tti1 degradation and promote survival in multiple myeloma
SCFFbxo9 and CK2 direct the cellular response to growth factor withdrawal via Tel2/Tti1 degradation and promote survival in multiple myeloma
The mTORC1 complex promotes protein translation and cell growth, whereas mTORC2 promotes survival. The Tel2 and Tt1 proteins belong to both complexes. Bassermann and colleagues demonstrate that following growth-factor deprivation, casein kinase 2 mediates phosphorylation of Tel2 and Tt1, specifically in the mTORC1 complex, to target them for degradation by the SCFFbxo9 ubiquitin ligase. This mechanism inactivates mTORC1 and activates mTORC2 and Akt signalling to promote survival of multiple myeloma cells.
- Vanesa Fernández-Sáiz
- , Bianca-Sabrina Targosz
- & Florian Bassermann
-
-
Article |
 Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass
Systems-wide analysis of ubiquitylation dynamics reveals a key role for PAF15 ubiquitylation in DNA-damage bypass
In a quantitative proteomics approach, Mailand, Choudhary and colleagues characterize ultraviolet-regulated ubiquitylation sites and identify a role for double mono-ubiquitylation of PCNA-associated factor PAF15 in bypassing replication-blocking lesions in DNA.
- Lou K. Povlsen
- , Petra Beli
- & Chunaram Choudhary
-
News & Views |
 Ubiquitin removal in the TGF-β pathway
Ubiquitin removal in the TGF-β pathway
The transforming growth factor (TGF-β) pathway is regulated by ubiquitin-mediated proteolysis at different levels. Two studies now identify deubiquitinating enzymes (DUBs) for the TGF-β type I receptor. Both ubiquitin-specific peptidase-4 (USP4) and -15 (USP15) extend the life of activated receptors against the negative pressure of receptor-ubiquitinating complexes, but through distinct modes of action.
- Kamna Aggarwal
- & Joan Massagué
-
-
Article |
 PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12
PRR5L degradation promotes mTORC2-mediated PKC-δ phosphorylation and cell migration downstream of Gα12
Wu and colleagues delineate an mTORC2-dependent cell migration pathway. They show that stimulation of the Gα12 protein subunit induces the ARAF/ERK-mediated expression of the RFFL E3 ubiquitin ligase. RFFL, in turn, targets the inhibitory PRR5L subunit of the mTORC2 complex for ubiquitylation and degradation, enabling mTORC2 to phosphorylate PKC-δ and promote cell migration.
- Xiaoqing Gan
- , Jiyong Wang
- & Dianqing Wu
-
Article |
 Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail
Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail
Integrin internalization through the endosomal pathway can lead either to recycling back to the surface or to lysosomal degradation. Faessler and colleagues now show that, following internalization, β1 integrins are bound by sorting nexin 17 in early endosomes to prevent integrin degradation in lysosomes and to promote surface recycling.
- Ralph Thomas Böttcher
- , Christopher Stremmel
- & Reinhard Fässler
-
Article |
 Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma
Fbxw7α- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma
The SCF ubiquitin ligase subunit Fbxw7 is a tumour suppressor that is mutated in many cancers. Pagano and colleagues now show that in multiple myeloma, Fbxw7α instead functions as a pro-survival factor by activating the NF-κB pathway through the ubiquitin-mediated degradation of p100, an NF-κB pathway inhibitor.
- Luca Busino
- , Scott E. Millman
- & Michele Pagano
-
Review Article |
 Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system
Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system
- Hemmo Meyer
- , Monika Bug
- & Sebastian Bremer
-
Article |
 APC/C-mediated multiple monoubiquitylation provides an alternative degradation signal for cyclin B1
APC/C-mediated multiple monoubiquitylation provides an alternative degradation signal for cyclin B1
Cyclin B is targeted for proteasome-mediated degradation by the E3 ligase APC/C, which is thought to generate polyubiquitin chains for the degradation of mitotic substrates. King and colleagues now demonstrate in Xenopus laevis extracts that multiple monoubiquitylation events are sufficient to target cyclin B1 for degradation.
- Nevena V. Dimova
- , Nathaniel A. Hathaway
- & Randall W. King
-
Letter |
 The LIMD1 protein bridges an association between the prolyl hydroxylases and VHL to repress HIF-1 activity
The LIMD1 protein bridges an association between the prolyl hydroxylases and VHL to repress HIF-1 activity
Oxygen levels regulate the stability of the transcription factor HIF-1 through the action of prolyl hydroxylases and the VHL ubiquitin ligase. Sharp and colleagues now identify a protein complex in which the Ajuba LIM-domain protein LIMD1 brings together prolyl hydroxylases and VHL to ensure efficient degradation of HIF-1.
- Daniel E. Foxler
- , Katherine S. Bridge
- & Tyson V. Sharp
-
Resource |
 Defining human ERAD networks through an integrative mapping strategy
Defining human ERAD networks through an integrative mapping strategy
Improperly folded proteins are targeted for destruction through the endoplasmic-reticulum-associated degradation pathway (ERAD). Kopito and colleagues present a high-resolution interaction analysis of the ERAD system in combination with functional genomics, and identify new ERAD components.
- John C. Christianson
- , James A. Olzmann
- & Ron R. Kopito
-
Article |
 ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation
ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation
Wallerian degeneration occurs in axons following cutting or crush injuries; however, the molecular mechanisms that regulate this process remain elusive. Araki and colleagues find that the ubiquitin ligase ZNRF1 promotes Wallerian degeneration by ubiquitylating AKT, which leads to increased GSK3B activity and subsequent inhibition of the tubulin-binding protein CRMP2.
- Shuji Wakatsuki
- , Fuminori Saitoh
- & Toshiyuki Araki
-
Letter |
 Caspase 8 inhibits programmed necrosis by processing CYLD
Caspase 8 inhibits programmed necrosis by processing CYLD
Caspase 8 is known to suppress necroptosis, but its relevant target protein was unknown. Ting and colleagues show that caspase 8 cleaves the deubiquitylase CYLD to inhibit necroptosis and promote cell survival.
- Marie Anne O’Donnell
- , Eva Perez-Jimenez
- & Adrian T. Ting
-
Letter |
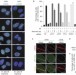 The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks
The ubiquitin-selective segregase VCP/p97 orchestrates the response to DNA double-strand breaks
The p97 AAA+ ATPase (also known as VCP) functions in various ubiquitin-regulated processes. Ramadan and colleagues now find that p97 is recruited to sites of DNA damage by Lys-48-linked ubiquitin chains, which are formed in a process mediated by RNF8. p97 then removes Lys-48–ubiquitin conjugates and promotes recruitment of DNA-repair factors.
- Mayura Meerang
- , Danilo Ritz
- & Kristijan Ramadan
-
News & Views |
 Misfolded proteins driven to destruction by Hul5
Misfolded proteins driven to destruction by Hul5
Misfolded proteins are potentially toxic and are therefore subjected to highly selective degradation by the ubiquitin–proteasome system. The identification of the Hul5 ubiquitin ligase as a major mediator of such 'quality-control' ubiquitylation following heat shock raises new questions about the design of these pathways.
- Daniel Finley
-
Article |
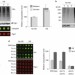 Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins
Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins
The ubiquitin–proteasome system clears misfolded proteins to maintain cellular homeostasis. Mayor and colleagues identify the ubiquitin ligase Hul5 as a critical component of the heat-shock response and show that it selectively targets misfolded cytosolic proteins for degradation.
- Nancy N. Fang
- , Alex H. M. Ng
- & Thibault Mayor
-
Letter |
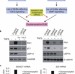 USP15 is a deubiquitylating enzyme for receptor-activated SMADs
USP15 is a deubiquitylating enzyme for receptor-activated SMADs
In the TGFβ pathway, receptor-activated SMADs (R-SMADs) associate with SMAD4 to regulate transcription. Piccolo and colleagues reveal that the deubiquitylase USP15 is required for TGFβ responses by reversing R-SMAD ubiquitylation and thereby promoting the retention of the SMAD complex at promoters.
- Masafumi Inui
- , Andrea Manfrin
- & Stefano Piccolo
-
Article |
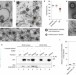 BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol
BAP31 and BiP are essential for dislocation of SV40 from the endoplasmic reticulum to the cytosol
Non-enveloped viruses such as SV40 are transported from the extracellular space into the host cell nucleus through a pathway involving endocytosis, trafficking to the endoplasmic reticulum (ER) lumen, transport across the ER membrane to the cytoplasm, and subsequent nuclear import. Helenius and colleagues provide insight into how SV40 escapes from the ER by showing that viral proteins interact with components of the host ER-associated degradation machinery (ERAD). These interactions are crucial for translocation of SV40 into the cytoplasm and infectivity.
- Roger Geiger
- , Daniel Andritschke
- & Ari Helenius

 New MAPS for misfolded proteins
New MAPS for misfolded proteins