News & Views |
Featured
-
-
Article |
 MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control
MIROs and DRP1 drive mitochondrial-derived vesicle biogenesis and promote quality control
By characterizing the composition of mitochondrial-derived vesicles (MDVs), König et al. define a MIRO1/2- and DRP1-dependent MDV biogenesis pathway and propose that MDVs maintain the mitochondrial proteome by shuttling assembled protein complexes to lysosomes.
- Tim König
- , Hendrik Nolte
- & Heidi M. McBride
-
News & Views |
 Mitochondrial UPR through generations
Mitochondrial UPR through generations
Neuronal mitochondria perturbation elicits a mitochondrial unfolded protein response (UPRmt) in peripheral tissues cell non-autonomously, dependent on the Wnt signalling pathway. A study now reveals that a Wnt-mediated increase in maternally inherited mitochondria DNA is responsible for transgenerational UPRmt induced by neuronal mitochondria perturbation.
- Mooncheol Park
- & Meng C. Wang
-
Article |
 The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels
The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels
Zhang et al. report that the systemic mitochondrial unfolded protein response triggered by neuronal mitochondrial stress can be transmitted across multiple generations in Caenorhabditis elegans via a mechanism involving elevation in mitochondrial DNA levels in oocytes.
- Qian Zhang
- , Zihao Wang
- & Ye Tian
-
News & Views |
 Protein quality and miRNA slicing get into phase
Protein quality and miRNA slicing get into phase
Phase separation can build assemblies and regulate biological function. Two articles link specific forms of protein and RNA degradation to phase separation. The polyubiquitin shuttle factor UBQLN2 localizes to stress granules where it may extract ubiquitinated proteins, and the miRISC complex functions through phase separation.
- Tanja Mittag
- & Nicolas L. Fawzi
-
-
News & Views |
 Hitchhiking on selective autophagy
Hitchhiking on selective autophagy
Selective autophagy is important for controlled degradation of cellular components. However, a selective autophagic degradation mechanism for ribosomes in mammals has remained unclear. A study now describes non-selective and selective ribosome degradation and a significant role for ‘bystander’ non-selective autophagy.
- Christian Münch
- & Ivan Dikic
-
Article |
 The MTM1–UBQLN2–HSP complex mediates degradation of misfolded intermediate filaments in skeletal muscle
The MTM1–UBQLN2–HSP complex mediates degradation of misfolded intermediate filaments in skeletal muscle
Gavriilidis et al. show that MTM1, which is mutated in X-linked centronuclear myopathy, and UBQLN2 recognize misfolded desmin and vimentin and trigger their degradation to clear misfolded intermediated filaments prior to aggregate formation.
- Christos Gavriilidis
- , Leila Laredj
- & Karim Hnia
-
News & Views |
 PERK links the clock and protein stress in cancer
PERK links the clock and protein stress in cancer
The unfolded protein response (UPR) regulates cell metabolism and survival in response to stress, yet how the UPR is connected to other signalling pathways is poorly understood. PERK is now shown to regulate Bmal1 and Clock proteins to promote cancer cell survival, revealing a link between growth regulation and circadian rhythms.
- Miguel Sanchez-Alvarez
- & Chris Bakal
-
Letter |
 Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy
Systematic analysis of ribophagy in human cells reveals bystander flux during selective autophagy
An and Harper quantify ribophagy in mammalian cells and show that nutrient-deprivation-induced ribophagy is independent of the ATG8 conjugation system, whereas proteotoxic stress-induced ribophagy requires ATG5 and VPS34.
- Heeseon An
- & J. Wade Harper
-
Article |
 A PERK–miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival
A PERK–miR-211 axis suppresses circadian regulators and protein synthesis to promote cancer cell survival
PERK regulates tumour cell survival. Bu et al. show that the unfolded protein response protein PERK induces miR-211 repression of the circadian factor Bmal1 to regulate protein synthesis and stress responses, contributing to tumour progression.
- Yiwen Bu
- , Akihiro Yoshida
- & J. Alan Diehl
-
Letter |
 Receptor oligomerization guides pathway choice between proteasomal and autophagic degradation
Receptor oligomerization guides pathway choice between proteasomal and autophagic degradation
Lu et al. show that the choice between proteasomal degradation and selective autophagy is independent of the ubiquitin-binding properties of the receptors but largely determined by oligomerization potential.
- Kefeng Lu
- , Fabian den Brave
- & Stefan Jentsch
-
News & Views |
 PARL paves the way to apoptosis
PARL paves the way to apoptosis
Although the mitochondrial inner membrane rhomboid peptidase PARL is known to participate in critical signalling cascades, its role in apoptosis has remained unclear. PARL is now shown to process the mitochondrial pro-apoptotic protein Smac (also known as DIABLO) for its subsequent release into the cytosol where it antagonizes XIAP-mediated caspase inhibition to promote apoptosis.
- Naotada Ishihara
- & Katsuyoshi Mihara
-
Article |
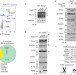 PARL mediates Smac proteolytic maturation in mitochondria to promote apoptosis
PARL mediates Smac proteolytic maturation in mitochondria to promote apoptosis
Saita et al. show that PARL cleaves Smac (also known as DIABLO) to generate an IAP-binding motif required for its apoptotic activity, identifying PARL-mediated Smac processing as a pro-apoptotic mitochondrial pathway.
- Shotaro Saita
- , Hendrik Nolte
- & Thomas Langer
-
News & Views |
 Targeting mutant p53 through the mevalonate pathway
Targeting mutant p53 through the mevalonate pathway
It is well established that mutant forms of the p53 tumour suppressor acquire pro-oncogenic activities. Inhibition of the mevalonate pathway is now shown to promote degradation of select oncogenic mutant p53 proteins, indicating that destabilization of mutant p53 could be a promising therapeutic strategy.
- William Freed-Pastor
- & Carol Prives
-
Article |
 DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway
DNAJA1 controls the fate of misfolded mutant p53 through the mevalonate pathway
Iwakuma and colleagues report that statins, through their action on the mevalonate pathway, lead to the ubiquitin-mediated degradation of misfolded mutant p53 by impairing its interaction with the Hsp40 family member, DNAJA1.
- Alejandro Parrales
- , Atul Ranjan
- & Tomoo Iwakuma
-
News & Views |
 New MAPS for misfolded proteins
New MAPS for misfolded proteins
Clearing misfolded proteins from the cytoplasm is essential to maintain cellular homeostasis. Now, a parallel clearance system is described that uses the deubiquitylase USP19 to enable secretion of misfolded cytoplasmic proteins when conventional proteasomal degradation is compromised. Misfolding-associated protein secretion (MAPS) has important implications for protein quality control and prion-like transmission.
- Norbert Volkmar
- , Emma Fenech
- & John C. Christianson
-
Article |
 IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation
IRE1α is an endogenous substrate of endoplasmic-reticulum-associated degradation
Through a proteomics approach, Qi and colleagues and Long and colleagues identify the sensor of the unfolded protein response IRE1α as an endogenous substrate of the E3 ubiquitin ligase involved in ER-associated degradation, Hrd1.
- Shengyi Sun
- , Guojun Shi
- & Ling Qi
-
News & Views |
 Q-bodies monitor the quinary state of the protein fold
Q-bodies monitor the quinary state of the protein fold
Cytoplasmic compartments containing misfolded proteins targeted for degradation, named Q-bodies, have been identified. Q-body formation is a dynamic process that actively manages the metastable state of the protein fold through small heat shock proteins and the Hsp70–Hsp90–Hsp110 proteostasis system to promote cellular fitness under both physiological and stress conditions.
- Daniela Martino Roth
- & William E. Balch
-
Article |
 Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress
Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress
Quality control of misfolded proteins is thought to involve proteasome-dependent degradation or, if this fails, sequestration into inclusion bodies. Frydman and colleagues reveal the existence of endoplasmic-reticulum-associated structures, termed Q-bodies, that concentrate misfolded proteins in a chaperone-dependent manner before degradation.
- Stéphanie Escusa-Toret
- , Willianne I. M. Vonk
- & Judith Frydman
-
Review Article |
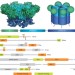 Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system
Emerging functions of the VCP/p97 AAA-ATPase in the ubiquitin system
- Hemmo Meyer
- , Monika Bug
- & Sebastian Bremer
-
News & Views |
 Misfolded proteins driven to destruction by Hul5
Misfolded proteins driven to destruction by Hul5
Misfolded proteins are potentially toxic and are therefore subjected to highly selective degradation by the ubiquitin–proteasome system. The identification of the Hul5 ubiquitin ligase as a major mediator of such 'quality-control' ubiquitylation following heat shock raises new questions about the design of these pathways.
- Daniel Finley
-
Article |
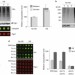 Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins
Hul5 HECT ubiquitin ligase plays a major role in the ubiquitylation and turnover of cytosolic misfolded proteins
The ubiquitin–proteasome system clears misfolded proteins to maintain cellular homeostasis. Mayor and colleagues identify the ubiquitin ligase Hul5 as a critical component of the heat-shock response and show that it selectively targets misfolded cytosolic proteins for degradation.
- Nancy N. Fang
- , Alex H. M. Ng
- & Thibault Mayor
-
Article |
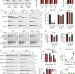 CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity
CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity
A chaperone complex containing CSPα, Hsc70 and SGT binds to monomeric SNAP-25 and prevents its aggregation and degradation. Loss of CSPα inhibits SNARE complex formation.
- Manu Sharma
- , Jacqueline Burré
- & Thomas C. Südhof

 Surveying the mitochondrial proteome
Surveying the mitochondrial proteome