Abstract
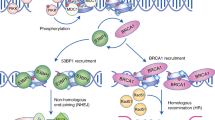
DNA double-strand breaks (DSBs) are perhaps the most toxic of all DNA lesions, with defects in the DNA-damage response to DSBs being associated with various human diseases. Although it is known that DSB repair pathways are tightly regulated by ubiquitylation, we do not yet have a comprehensive understanding of how deubiquitylating enzymes (DUBs) function in DSB responses. Here, by carrying out a multidimensional screening strategy for human DUBs, we identify several with hitherto unknown links to DSB repair, the G2/M DNA-damage checkpoint and genome-integrity maintenance. Phylogenetic analyses reveal functional clustering within certain DUB subgroups, suggesting evolutionally conserved functions and/or related modes of action. Furthermore, we establish that the DUB UCHL5 regulates DSB resection and repair by homologous recombination through protecting its interactor, NFRKB, from degradation. Collectively, our findings extend the list of DUBs promoting the maintenance of genome integrity, and highlight their potential as therapeutic targets for cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hoeijmakers, J. H. Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 (2001).
Khanna, K. K. & Jackson, S. P. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 27, 247–254 (2001).
Kastan, M. B. DNA damage responses: mechanisms and roles in human disease: 2007 G.H.A. Clowes Memorial Award Lecture. Mol. Cancer Res. 6, 517–524 (2008).
Shiloh, Y. The ATM-mediated DNA-damage response: taking shape. Trends Biochem. Sci. 31, 402–410 (2006).
Jackson, S. P. Sensing and repairing DNA double-strand breaks. Carcinogenesis 23, 687–696 (2002).
Jackson, S. P. & Durocher, D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol. Cell 49, 795–807 (2013).
Kerscher, O., Felberbaum, R. & Hochstrasser, M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu. Rev. Cell Biol. 22, 159–180 (2006).
Komander, D. & Rape, M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012).
Reyes-Turcu, F. E., Ventii, K. H. & Wilkinson, K. D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 78, 363–397 (2009).
Komander, D., Clague, M. J. & Urbe, S. Breaking the chains: structure and function of the deubiquitinases. Nat. Rev. Mol. Cell Biol. 10, 550–563 (2009).
Khoronenkova, S. V. et al. ATM-dependent downregulation of USP7/HAUSP by PPM1G activates p53 response to DNA damage. Mol. Cell 45, 801–813 (2012).
Nakajima, S. et al. Ubiquitin-specific protease 5 is required for the efficient repair of DNA double-strand breaks. PLoS ONE 9, e84899 (2014).
Nicassio, F. et al. Human USP3 is a chromatin modifier required for S phase progression and genome stability. Curr. Biol. 17, 1972–1977 (2007).
Nijman, S. M. et al. The deubiquitinating enzyme USP1 regulates the Fanconi anemia pathway. Mol. Cell 17, 331–339 (2005).
Nishikawa, H. et al. BRCA1-associated protein 1 interferes with BRCA1/BARD1 RING heterodimer activity. Cancer Res. 69, 111–119 (2009).
Sobhian, B. et al. RAP80 targets BRCA1 to specific ubiquitin structures at DNA damage sites. Science 316, 1198–1202 (2007).
Wiltshire, T. D. et al. Sensitivity to poly(ADP-ribose) polymerase (PARP) inhibition identifies ubiquitin-specific peptidase 11 (USP11) as a regulator of DNA double-strand break repair. J. Biol. Chem. 285, 14565–14571 (2010).
Yu, H. et al. Tumor suppressor and deubiquitinase BAP1 promotes DNA double-strand break repair. Proc. Natl Acad. Sci. USA 111, 285–290 (2014).
Polo, S. E. & Jackson, S. P. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes. Dev. 25, 409–433 (2011).
Yoshida, A., Yoneda-Kato, N. & Kato, J. Y. CSN5 specifically interacts with CDK2 and controls senescence in a cytoplasmic cyclin E-mediated manner. Sci. Rep. 3, 1054 (2013).
Burrows, A. C., Prokop, J. & Summers, M. K. Skp1-Cul1-F-box ubiquitin ligase (SCF(βTrCP))-mediated destruction of the ubiquitin-specific protease USP37 during G2-phase promotes mitotic entry. J. Biol. Chem. 287, 39021–39029 (2012).
Aressy, B. et al. A screen for deubiquitinating enzymes involved in the G(2)/M checkpoint identifies USP50 as a regulator of HSP90-dependent Wee1 stability. Cell Cycle 9, 3815–3822 (2010).
Matsuoka, S. et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 316, 1160–1166 (2007).
Nakada, S. et al. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature 466, 941–946 (2010).
Mosbech, A., Lukas, C., Bekker-Jensen, S. & Mailand, N. The deubiquitylating enzyme USP44 counteracts the DNA double-strand break response mediated by the RNF8 and RNF168 ubiquitin ligases. J. Biol. Chem. 288, 16579–16587 (2013).
Kato, K. et al. Fine-tuning of DNA damage-dependent ubiquitination by OTUB2 supports the DNA repair pathway choice. Mol. Cell 53, 617–630 (2014).
Yao, T. et al. Proteasome recruitment and activation of the Uch37 deubiquitinating enzyme by Adrm1. Nat. Cell Biol. 8, 994–1002 (2006).
Sartori, A. A. et al. Human CtIP promotes DNA end resection. Nature 450, 509–514 (2007).
Gravel, S., Chapman, J. R., Magill, C. & Jackson, S. P. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes. Dev. 22, 2767–2772 (2008).
Qiu, X. B. et al. hRpn13/ADRM1/GP110 is a novel proteasome subunit that binds the deubiquitinating enzyme, UCH37. EMBO J. 25, 5742–5753 (2006).
Hamazaki, J. et al. A novel proteasome interacting protein recruits the deubiquitinating enzyme UCH37 to 26S proteasomes. EMBO J. 25, 4524–4536 (2006).
Lam, Y. A., Xu, W., DeMartino, G. N. & Cohen, R. E. Editing of ubiquitin conjugates by an isopeptidase in the 26S proteasome. Nature 385, 737–740 (1997).
Jacquemont, C. & Taniguchi, T. Proteasome function is required for DNA damage response and fanconi anemia pathway activation. Cancer Res. 67, 7395–7405 (2007).
Murakawa, Y. et al. Inhibitors of the proteasome suppress homologous DNA recombination in mammalian cells. Cancer Res. 67, 8536–8543 (2007).
Mailand, N. et al. RNF8 ubiquitylates histones at DNA double-strand breaks and promotes assembly of repair proteins. Cell 131, 887–900 (2007).
Kolas, N. K. et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318, 1637–1640 (2007).
Huen, M. S. et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell 131, 901–914 (2007).
Yao, T. et al. Distinct modes of regulation of the Uch37 deubiquitinating enzyme in the proteasome and in the Ino80 chromatin-remodeling complex. Mol. Cell 31, 909–917 (2008).
Gospodinov, A., Tsaneva, I. & Anachkova, B. RAD51 foci formation in response to DNA damage is modulated by TIP49. Int. J. Biochem. Cell Biol. 41, 925–933 (2009).
Gospodinov, A. et al. Mammalian Ino80 mediates double-strand break repair through its role in DNA end strand resection. Mol. Cell. Biol. 31, 4735–4745 (2011).
Park, E. J., Hur, S. K. & Kwon, J. Human INO80 chromatin-remodelling complex contributes to DNA double-strand break repair via the expression of Rad54B and XRCC3 genes. Biochem. J. 431, 179–187 (2010).
Wu, S. et al. A YY1-INO80 complex regulates genomic stability through homologous recombination-based repair. Nat. Struct. Mol. Biol. 14, 1165–1172 (2007).
Chen, L. et al. Subunit organization of the human INO80 chromatin remodeling complex: an evolutionarily conserved core complex catalyzes ATP-dependent nucleosome remodeling. J. Biol. Chem. 286, 11283–11289 (2011).
Seeber, A., Hauer, M. & Gasser, S. M. Nucleosome remodelers in double-strand break repair. Curr. Opin. Genet. Dev. 23, 174–184 (2013).
Min, J. N. et al. The mINO80 chromatin remodeling complex is required for efficient telomere replication and maintenance of genome stability. Cell Res. 23, 1396–1413 (2013).
Aymard, F. et al. Transcriptionally active chromatin recruits homologous recombination at DNA double-strand breaks. Nat. Struct. Mol. Biol. 21, 366–374 (2014).
Kolas, N. K. et al. Orchestration of the DNA-damage response by the RNF8 ubiquitin ligase. Science 318, 1637–1640 (2007).
Galanty, Y. et al. Mammalian SUMO E3-ligases PIAS1 and PIAS4 promote responses to DNA double-strand breaks. Nature 462, 935–939 (2009).
Huertas, P. & Jackson, S. P. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J. Biol. Chem. 284, 9558–9565 (2009).
Sakaue-Sawano, A. et al. Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498 (2008).
Galanty, Y., Belotserkovskaya, R., Coates, J. & Jackson, S. P. RNF4, a SUMO-targeted ubiquitin E3 ligase, promotes DNA double-strand break repair. Genes Dev. 26, 1179–1195 (2012).
Certo, M. T. et al. Tracking genome engineering outcome at individual DNA breakpoints. Nat. Methods 8, 671–676 (2011).
Forment, J. V., Walker, R. V. & Jackson, S. P. A high-throughput, flow cytometry-based method to quantify DNA-end resection in mammalian cells. Cytometry A 81, 922–928 (2012).
Acknowledgements
We thank all members of the S.P.J. laboratory for helpful discussions. We are grateful to A. Blackford, D. Larrieu and K. Dry for commenting on the manuscript, N. Lawrence and A. Sossick for help with microscopy, and C. Green for scientific advice. Research in the S.P.J. laboratory is funded by Cancer Research UK Program Grant C6/A11224, the European Research Council and the European Community Seventh Framework Program grant agreement no. HEALTH-F2-2010-259893 (DDResponse). Core infrastructure funding was provided by Cancer Research UK Grant C6946/A14492 and Wellcome Trust Grant WT092096. S.P.J. receives a salary from the University of Cambridge, supplemented by Cancer Research UK. R.N. was funded by a Daiichi Sankyo Foundation of Life Science fellowship and Cancer Research UK Project Grant C6/A14831; C.l.S. was funded by European Molecular Biology Organization Grant ALTF 1165-2010. J.V.F. was supported by Cancer Research UK Program Grant C6/A11224 and the Ataxia Telangiectasia Society.
Author information
Authors and Affiliations
Contributions
R.N. designed experiments through discussion with P.W., J.T., C.l.S., Y.G. and S.P.J.; P.W., R.N., M.J.C. and S.U. cloned human DUBs. R.N., P.W., C.l.S. and J.T. carried out the screens. P.W. and C.l.S. carried out cell cycle analyses. R.N. carried out most of the other studies with P.W.’s assistance. R.N. and S.P.J. wrote the paper. All other authors, especially P.W., commented and suggested revisions for the paper.
Corresponding author
Ethics declarations
Competing interests
S.P.J. is a founder and shareholder of MISSION Therapeutics Ltd., which is developing DUB inhibitors for therapeutic applications. The other authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 UCHL5 is dispensable for resection initiation but required for full resection.
(a) Cells transfected with the indicated siRNAs were subjected to cell cycle profile analysis. Data show the means of three biologically independent experiments. The error bars indicate standard error of means. (b) Clonogenic survival assay with camptothecin (CPT). Depletion of CtIP (siCtIP) is a positive control. Data show the means of two biologically independent experiments. (c) Stable cell lines expressing GFP or GFP–UCHL5 (WT or DD) were processed for immunoblotting with indicated antibodies. Tubulin is used as a loading control. Protein levels of GFP and GFP–UCHL5 derivatives are indicated below. The modified bands detected with GFP–UCHL5 (DD) are ubiquitylated UCHL5 as revealed by IP-western blotting studies (data not shown). (d,e,f,g) Cells transfected with indicated siRNAs were treated with 1 μM of CPT for 1 h and processed for immunofluorescent staining with indicated antibodies. Nuclei were stained with DAPI. Depletion of CtIP (siCtIP) is a positive control. (f) Quantification of data shown in e. Proportion of cells with >10 RPA foci in gH2AX positive cells were calculated by counting 100 gH2AX positive cells per experiment. Data show the means of two biologically independent experiments. (h) Kinetics of GFP–CtIP accumulation at DNA damage sites was assessed in cells transfected with indicated siRNAs. Signal intensity of GFP–CtIP at DNA damage sites relative to the unirradiated area was quantified Data show the means of two biologically independent experiments. (i) Cells transfected with the individual or indicated combinations of siRNAs were processed for quantitative resection assays. Data show the means of two biologically independent experiments. Scale bars indicate 10 μm.
Supplementary Figure 2 UCHL5 depletion does not result in general proteasome dysfunction.
(a) Cells treated with indicated siRNAs or proteasome inhibitor MG132 (10 μM for 6 h) were processed for immunoblotting with indicated antibodies. (b) U2OS cells stably expressing GFP-53BP1 transfected with indicated siRNAs were mock irradiated (−IR) or irradiated with 5 Gy of IR followed by 1 h incubation (+IR). Depletion of RNF8 or PSMD14 and MG132 treatment (10 μM for 1 h) were positive controls. Scale bar indicates 10 μm. (c) Quantitative resection assay with MG132 treatment (10 μM for 1 h). Data show the means of two biologically independent experiments. (d) Quantitative resection assay with indicated siRNAs. Data show the means of two biologically independent experiments. (e) Cells treated with indicated siRNAs or MG132 (10 μM for 6 h) were processed for immunoblotting with indicated antibodies.
Supplementary Figure 3 UCHL5 protects NFRKB from proteasomal turnover.
(a) GFP or GFP–UCHL5 (WT or DD) expressing cells were subjected to immunoprecipitation with an anti-GFP antibody, followed by immunoblotting with the indicated antibodies. (b) Cells transfected with indicated siRNAs were subjected to RT-qPCR for NFRKB mRNA. NFRKB depletion (siNFRKB) is a positive control. Data show the means of two biologically independent experiments. (c) Cells transfected with indicated siRNAs were processed for immunoblotting with indicated antibodies. (d) GFP–NFRKB expressing cells transfected with indicated siRNAs were treated or mock treated with MG132 (10 μM for 6 h) and processed for immunoblotting with indicated antibodies. Brackets indicate modified forms of NFRKB. Tubulin and histone H2AX are loading controls. (e) Stable cell lines expressing GFP or GFP–NFRKB were processed for immunoblotting with indicated antibodies. (f) Input fractions corresponding to Fig. 7d. (g) In vitro deubiquitylation assay with recombinant GST-tagged UCHL5 and human cell derived NFRKB modified with HA-tagged ubiquitin.
Supplementary Figure 4 NFRKB depletion results in defective resection similar to UCHL5 depletion.
(a) Stable cell lines expressing GFP or GFP–NFRKB were transfected with indicated siRNAs and subjected to immunoblotting analysis with indicated antibodies. (b,c,d) U2OS cells transfected with indicated siRNAs were treated with CPT (1 μM for 1 h) and subjected to immunofluorescent staining with indicated antibodies (b,c) or quantitative DNA-end resection assays with anti-RPA2 antibody. Data show the means of two biologically independent experiments. (d) Scale bar indicates 10 μm. (c) Quantification of data shown in b. Population of cells with >10 RPA foci in γH2AX positive cells were calculated by counting 100 γH2AX positive cells per experiment Data show the means of two biologically independent experiments. (e) U2OS cells transfected with indicated siRNAs were irradiated with 5 Gy IR and 8 h after irradiation, subjected to immunofluorescent staining with anti-γH2AX and anti-RAD51 antibodies. Populations of cells with >5 RAD51 foci colocalized with γH2AX were plotted by counting 100 cells per experiment. Data show the means of two biologically independent experiments. (f) U2OS cells transfected with indicated siRNAs were treated with CPT (1 μM for 1 h) and subjected to immunoblotting analysis with indicated antibodies. (g) Cells transfected with indicated siRNAs were subjected to cell cycle profile analysis. Data show the means of two biologically independent experiments. (h) U2OS cells transfected with indicated siRNAs were processed for immnuoblotting with indicated antibodies. (i) U2OS cells transfected with indicated combinations of siRNAs were processed for immnuoblotting with indicated antibodies. (j) U2OS cells transfected with indicated siRNAs were treated with 1 μM of CPT for 1 h and processed for immunoblotting with indicated antibodies. (k) Kinetics of GFP–EXO1 accumulation at DNA damage sites was assessed in cells transfected with indicated siRNAs. Signal intensity of GFP–EXO1 at DNA damage sites relative to the unirradiated area was quantified. Data show the means of two biologically independent experiments. (l) Input fractions corresponding to Fig. 7k. Arrows indicate NFRKB protein band. Tubulin is used as a loading control.
Supplementary information
Supplementary Information
Supplementary Information (PDF 929 kb)
Rights and permissions
About this article
Cite this article
Nishi, R., Wijnhoven, P., le Sage, C. et al. Systematic characterization of deubiquitylating enzymes for roles in maintaining genome integrity. Nat Cell Biol 16, 1016–1026 (2014). https://doi.org/10.1038/ncb3028
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb3028