Article
|
Open Access
Featured
-
-
Article
| Open Access Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors
Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors
Visible light-induced glycosylation reactions are achieved by either photoactivating a photosensitizer or using a stoichiometric activator, while glycosylation via a photoactive glycosyl donor was so far not reported. In this study, the authors develop a photosensitizer free visible-light-mediated glycosylation approach using photoactive 2-glycosyloxy tropone as the donor, obtaining a wide range of O-glycosides or oligosaccharides.
- Jing Zhang
- , Zhao-Xiang Luo
- & De-Cai Xiong
-
Article
| Open Access Selective C(sp3)–H arylation/alkylation of alkanes enabled by paired electrocatalysis
Selective C(sp3)–H arylation/alkylation of alkanes enabled by paired electrocatalysis
The combination of electrochemistry and photochemistry in the context of organic synthesis is in its infancy. Here, the authors report selective C(sp3)–H arylation/alkylation of alkanes, using Earth-abundant bimetallic transition metal catalysis, under photo- and electrochemical conditions.
- Long Zou
- , Siqi Xiang
- & Qingquan Lu
-
Article
| Open Access Selective ring expansion and C−H functionalization of azulenes
Selective ring expansion and C−H functionalization of azulenes
Fused carbocycles are key structural elements of molecules in nature and they are often found in drugs and organic materials, but bicyclic systems containing six and seven-membered rings are difficult to prepare. Here, the authors functionalize an azulene skeleton that consists of fused five and seven-membered rings and carry out a ring expansion reaction to afford the desired bicycles.
- Sangjune Park
- , Cheol-Eui Kim
- & Phil Ho Lee
-
Article
| Open Access Nickel/photoredox dual catalyzed arylalkylation of nonactivated alkenes
Nickel/photoredox dual catalyzed arylalkylation of nonactivated alkenes
Dicarbofunctionalization of nonactivated alkenes is a simple way to rapidly build molecular complexity, but these transformations have not been extensively explore under metallaphotoredox conditions. Here, the authors develop a nickel/photoredox dual catalytic system for arylalkylation of alkenes.
- Yuxi Gao
- , Lijuan Gao
- & Chengfeng Xia
-
Article
| Open Access Ligand-modulated nickel-catalyzed regioselective silylalkylation of alkenes
Ligand-modulated nickel-catalyzed regioselective silylalkylation of alkenes
Multicomponent cross-coupling of alkenes with silicon reagents is used to yield complex silicon-containing compounds from readily accessible feedstock chemicals but the reaction with simple alkenes remains challenging. Here, the authors report a regioselective silylalkylation of simple alkenes, which is enabled by using a stable Ni(II) salt and an inexpensive trans-1,2-diaminocyclohexane ligand as a catalyst.
- Chao Ding
- , Yaoyu Ren
- & Guoyin Yin
-
Article
| Open Access Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis
Crown-hydroxylamines are pH-dependent chelating N,O-ligands with a potential for aerobic oxidation catalysis
Despite the rich coordination chemistry, hydroxylamines are rarely used as ligands for transition metal coordination compounds. Here the authors designed macrocyclic poly-N-hydroxylamines that form complexes containing a d-metal ion and coordinate by up to six hydroxylamine fragments.
- Vladislav K. Lesnikov
- , Ivan S. Golovanov
- & Alexey Yu. Sukhorukov
-
Article
| Open Access Modular access to alkylgermanes via reductive germylative alkylation of activated olefins under nickel catalysis
Modular access to alkylgermanes via reductive germylative alkylation of activated olefins under nickel catalysis
While carbon-introducing difunctionalization of C-C double bonds is well established, the analogous difunctionalization for introducing germanium group and other functionalities remains elusive. Here, the authors describe a nickel-catalyzed germylative alkylation of activated olefins with easily accessible primary, secondary and tertiary alkyl bromides and chlorogermanes as the electrophiles to form C-Ge and C-Calkyl bonds simultaneously.
- Rui Gu
- , Xiujuan Feng
- & Xuan Zhang
-
Article
| Open Access Mechanistic study on the side arm effect in a palladium/Xu-Phos-catalyzed enantioselective alkoxyalkenylation of γ-hydroxyalkenes
Mechanistic study on the side arm effect in a palladium/Xu-Phos-catalyzed enantioselective alkoxyalkenylation of γ-hydroxyalkenes
Recently, the asymmetric bifunctionalization of alkenes has received much attention but the development of enantioselective alkoxyalkenylation has posed a considerable challenge and has lagged largely behind. Here, the authors report a palladium-catalyzed enantioselective alkoxyalkenylation reaction, using a range of primary, secondary, and tertiary γ-hydroxyalkenes with alkenyl halides.
- Shuai Zhu
- , Zihao Ye
- & Junliang Zhang
-
Article
| Open Access Nickel-catalyzed direct stereoselective α-allylation of ketones with non-conjugated dienes
Nickel-catalyzed direct stereoselective α-allylation of ketones with non-conjugated dienes
Low-valent nickel(0) complexes that promote α-functionalization of carbonyls leveraging its pro-nucleophilic character in conjunction with olefin acceptors are very scarce despite their attractivity. Here, the authors report a Ni(0)NHC catalyst which converts ketones and non-conjugated dienes to synthetically highly valuable α-allylated products.
- Yi-Xuan Cao
- , Matthew D. Wodrich
- & Nicolai Cramer
-
Article
| Open Access Stereoselectivity control in Rh-catalyzed β-OH elimination for chiral allene formation
Stereoselectivity control in Rh-catalyzed β-OH elimination for chiral allene formation
The chiral allene formation reactions catalyzed by palladium occur via a syn elimination mechanism, but there is a lack in understanding of the stereoselectivity in rhodium catalyzed reactions, specifically the β-OH elimination step. Here, the authors report the sterically controlled Rh-catalyzed SN2’-type substitution reactions of tertiary propargylic alcohols with arylmetallic species affording the non-readily available enantioenriched tetrasubstituted allenes via either exclusive syn- or anti-β-OH elimination.
- Jie Wang
- , Wei-Feng Zheng
- & Shengming Ma
-
Article
| Open Access Facile access to benzofuran derivatives through radical reactions with heteroatom-centered super-electron-donors
Facile access to benzofuran derivatives through radical reactions with heteroatom-centered super-electron-donors
The use of heteroatom-centered anions as super-electron-donors (SEDs) for direct single-electron-transfer reactions has rarely been studied. Here, the authors show that heteroatom anions can be applied as SEDs to initiate radical reactions for synthesis of 3-substituted benzofurans, which have applications in both organic synthesis and pesticide development.
- Shichun Jiang
- , Wei Wang
- & Yonggui Robin Chi
-
Article
| Open Access Synthesis of dienes from pyrrolidines using skeletal modification
Synthesis of dienes from pyrrolidines using skeletal modification
Saturated N-heterocyclic pyrrolidines are common in natural products and medicinal compounds, but reconstruction of their skeletal structures to access new chemical space is a challenging. Here, the authors report a skeletal modification strategy for conversion of polar cyclic pyrrolidines into nonpolar linear dienes through a N-atom removal and deconstruction process.
- Haitao Qin
- , Ting Guo
- & Hongjian Lu
-
Article
| Open Access Thermo-responsive chiral micelles as recyclable organocatalyst for asymmetric Rauhut-Currier reaction in water
Thermo-responsive chiral micelles as recyclable organocatalyst for asymmetric Rauhut-Currier reaction in water
Developing eco-friendly chiral organocatalysts with the combined advantages of homogeneous catalysis and heterogeneous processes is challenging. Here, the authors present, a family of amphiphilic one handed helical polyisocyanides bearing phosphine pendants which self-assembled into well-defined chiral micelles in water and show thermo-responsiveness
- Lei Xu
- , Li Zhou
- & Zong-Quan Wu
-
Article
| Open Access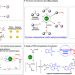 Bifunctionality of dirhodium tetracarboxylates in metallaphotocatalysis
Bifunctionality of dirhodium tetracarboxylates in metallaphotocatalysis
Traditional metallaphotocatalysis often requires two or more separate catalysts and is considered to be costly and not tolerant towards a wide substrate scope. Here the authors realize metallaphotocatalysis with a bifunctional dirhodium tetracarboxylate as single catalyst component to merge carbenoid chemistry and 1O2 chemistry.
- Taoda Shi
- , Tianyuan Zhang
- & Wenhao Hu
-
Article
| Open Access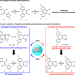 Selection of isomerization pathways of multistep photoswitches by chalcogen bonding
Selection of isomerization pathways of multistep photoswitches by chalcogen bonding
Multistep photoswitches can engage in different photoisomerization pathways, but are challenging to control. Here, the authors report the development of multistep photoswitches by manipulation of the strength and mechanism of chalcogen bonding interactions.
- Shuaipeng Jia
- , Hebo Ye
- & Lei You
-
Article
| Open Access Nucleophilic aromatization of monoterpenes from isoprene under nickel/iodine cascade catalysis
Nucleophilic aromatization of monoterpenes from isoprene under nickel/iodine cascade catalysis
Organic compounds possessing two isoprene units play important roles in chemical industry. Herein, the authors use bulk C5 chemical—isoprene to synthesise various monoterpenoids via a nucleophilic aromatization of monoterpenes under cascade catalysis of nickel and iodine
- Wei-Song Zhang
- , Ding-Wei Ji
- & Qing-An Chen
-
Article
| Open Access Double boron–oxygen-fused polycyclic aromatic hydrocarbons: skeletal editing and applications as organic optoelectronic materials
Double boron–oxygen-fused polycyclic aromatic hydrocarbons: skeletal editing and applications as organic optoelectronic materials
The development of chemically diverse boron-oxygen-fused polycyclic aromatic hydrocarbons has been limited by their synthetic complexity. Here, authors report a one-pot strategy for their facile synthesis and demonstrate their potential as ultralong afterglow and host materials for deep-blue OLEDs.
- Guijie Li
- , Kewei Xu
- & Yuan-Bin She
-
Article
| Open Access Asymmetric formal C–C bond insertion into aldehydes via copper-catalyzed diyne cyclization
Asymmetric formal C–C bond insertion into aldehydes via copper-catalyzed diyne cyclization
The formal C–C bond insertion into aldehydes is an attractive methodology for the assembly of homologated carbonyl compounds but the homologation of aldehydes has been limited to diazo approach and the enantioselective reaction was rarely developed. Here, the authors report an asymmetric formal C–C bond insertion into aldehydes through diyne cyclization strategy
- Cui-Ting Li
- , Lin-Jun Qi
- & Bo Zhou
-
Article
| Open Access Synthesis of tertiary alkylphosphonate oligonucleotides through light-driven radical-polar crossover reactions
Synthesis of tertiary alkylphosphonate oligonucleotides through light-driven radical-polar crossover reactions
Alkylphosphonates are used as chargeneutral replacements for naturally-occurring phosphodiester backbones in nucleotides but the choice of alkyl moieties that can be attached to phosphorus atoms in these compounds is limited to methyl groups or primary/secondary alkyls. Here, the authors demonstrate the tertiary alkylation of the phosphorus atoms of phosphites bearing two 2’-deoxynuclosides using a carbocation generated via a light-driven radical-polar crossover mechanism
- Kenji Ota
- , Kazunori Nagao
- & Hirohisa Ohmiya
-
Article
| Open Access Nickel-catalyzed acylzincation of allenes with organozincs and CO
Nickel-catalyzed acylzincation of allenes with organozincs and CO
The development of carbonylative reactions with CO gas under mild conditions are important in organic synthesis. Here, the authors demonstrate an earth-abundant nickel-catalyzed three-component tandem acylzincation/cyclization sequence of allene and alkylzinc reagent with 1 atm of CO.’
- Xianqing Wu
- , Chenglong Wang
- & Yifeng Chen
-
Article
| Open Access Organomediated electrochemical fluorosulfonylation of aryl triflates via selective C–O bond cleavage
Organomediated electrochemical fluorosulfonylation of aryl triflates via selective C–O bond cleavage
Although aryl triflates are essential building blocks in organic synthesis, the applications as aryl radical precursors are limited. Here, the authors report an organomediated electrochemical strategy for the generation of aryl radicals from aryl triflates, providing a method for the synthesis of aryl sulfonyl fluorides from feedstock phenol derivatives under very mild conditions.
- Xianqiang Kong
- , Yiyi Chen
- & Zhong-Yan Cao
-
Article
| Open Access A tautomerized ligand enabled meta selective C–H borylation of phenol
A tautomerized ligand enabled meta selective C–H borylation of phenol
Remote meta selective C–H Functionalization of aromatic compounds remains challenging in chemical synthesis. Here, the authors report an iridium catalyst bearing a bidentate pyridine-pyridone ligand framework that efficiently catalyzes this meta selective borylation reaction.
- Saikat Guria
- , Mirja Md Mahamudul Hassan
- & Buddhadeb Chattopadhyay
-
Article
| Open Access Electrochemical halogen-atom transfer alkylation via α-aminoalkyl radical activation of alkyl iodides
Electrochemical halogen-atom transfer alkylation via α-aminoalkyl radical activation of alkyl iodides
Alkyl halides, widely recognized as important building blocks and reagents in organic synthesisbut generating alkyl radicals directly from unactivated alkyl halides under mild conditions remains a challenge. Here the authors report an effective electrooxidation strategy for generating alkyl radicals from unactivated alkyl iodides via an electrochemical halogen-atom transfer process under mild conditions
- Xiang Sun
- & Ke Zheng
-
Article
| Open Access Stapling strategy for slowing helicity interconversion of α-helical peptides and isolating chiral auxiliary-free one-handed forms
Stapling strategy for slowing helicity interconversion of α-helical peptides and isolating chiral auxiliary-free one-handed forms
In nature, α-helical peptides adopt right-handed conformations dictated by L-amino acids, but isolating one-handed α-helical peptides composed of only achiral components remains a challenge. Here, the authors achieve this by optical resolution of the corresponding racemic (quasi-)static α-helical peptide with double stapling, which effectively freezes the interconversion between the right-handed (P)- and left-handed (M)-α-helices.
- Naoki Ousaka
- , Mark J. MacLachlan
- & Shigehisa Akine
-
Article
| Open Access Cu-catalyzed asymmetric regiodivergent electrosynthesis and its application in the enantioselective total synthesis of (-)-fumimycin
Cu-catalyzed asymmetric regiodivergent electrosynthesis and its application in the enantioselective total synthesis of (-)-fumimycin
Quaternary amino acids are important building blocks and precursors of medicinal compounds. Here, the authors describe a copper-catalyzed regiodivergent electrochemical core structure-oriented crossdehydrogenative coupling reaction of Schiff bases and commercially available hydroquinones to obtain three classes of chiral quaternary amino acid derivatives
- Tian Xie
- , Jianming Huang
- & Chang Guo
-
Article
| Open Access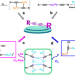 Anaerobic photoinduced Cu(0/I)-mediated Glaser coupling in a radical pathway
Anaerobic photoinduced Cu(0/I)-mediated Glaser coupling in a radical pathway
The reaction mechanism of the historic copper-catalyzed Glaser coupling has been debated to be based on redox cycles of Cu ions in specific oxidation states or on a radical mechanism based on Cu(0)/Cu(I). Here, the authors demonstrate two coexisting Glaser coupling pathways which can be differentiated by anaerobic/irradiation or aerobic reaction conditions
- Siqi Zhang
- & Liang Zhao
-
Article
| Open Access Mechanistic insights into excited-state palladium catalysis for C–S bond formations and dehydrogenative sulfonylation of amines
Mechanistic insights into excited-state palladium catalysis for C–S bond formations and dehydrogenative sulfonylation of amines
Photocatalytic selective C(sp3)–H activation/cross-coupling reactions are appealing in organic synthesis. Here the authors describe the development of excited-state Pd-catalyzed dehydrogenative β-sulfonylation reactions using amines and aryl sulfonyl chlorides via intermolecular hydrogen atom transfer and C−S cross-coupling processes at room temperature.
- Krishnamoorthy Muralirajan
- , Rajesh Kancherla
- & Magnus Rueping
-
Article
| Open Access Photoelectrochemical oxidative C(sp3)−H borylation of unactivated hydrocarbons
Photoelectrochemical oxidative C(sp3)−H borylation of unactivated hydrocarbons
Organoboron compounds are of high interest in organic synthesis due to the versatility of boryl substituents that allows access to further modifications. Here, the authors report on the development of an oxidative C(sp3)−H borylation reaction under metal- and oxidant-free conditions, enabled by photoelectrochemical strategy
- Ping-Fu Zhong
- , Jia-Lin Tu
- & Wujiong Xia
-
Article
| Open Access Metal-free electrochemical dihydroxylation of unactivated alkenes
Metal-free electrochemical dihydroxylation of unactivated alkenes
Methods to construct 1,2-diols have previously been developed, but often require the participation of a metal and are limited to activated alkenes. Here, the authors report a metal-free electrochemical dihydroxylation of unactivated alkenes providing valuable and versatile dihydroxylated products.
- Min Liu
- , Tian Feng
- & Youai Qiu
-
Article
| Open Access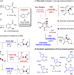 Catalytic 4-exo-dig carbocyclization for the construction of furan-fused cyclobutanones and synthetic applications
Catalytic 4-exo-dig carbocyclization for the construction of furan-fused cyclobutanones and synthetic applications
Aromatic ring fused cyclobutanone is a strained motif with broad applications. Here, the authors report a catalytic 4- exo-dig process, which proved successful to access furan-fused cyclobutanones that can serve as versatile synthetic blocks.
- Kemiao Hong
- , Yi Zhou
- & Xinfang Xu
-
Article
| Open Access Visible light-triggered selective C(sp2)-H/C(sp3)-H coupling of benzenes with aliphatic hydrocarbons
Visible light-triggered selective C(sp2)-H/C(sp3)-H coupling of benzenes with aliphatic hydrocarbons
The direct and selective coupling between unactivated C(sp2)-H and C(sp3)-H bonds is widely recognized as one of the most challenging tasks in organic synthesis. Here, the authors present an iron halidepromoted photochemical aerobic oxidation strategy for achieving such transformations.
- Qian-Yu Li
- , Shiyan Cheng
- & Lei Gong
-
Article
| Open Access Supramolecular catalysis with ethers enabled by dual chalcogen bonding activation
Supramolecular catalysis with ethers enabled by dual chalcogen bonding activation
The activation of ethers by weak interactions in supramolecular catalysis remains challenging. Here, the authors describe an activation mode based on dual Se···π and Se···O bonding, that can activate benzylic as well as allylic ether C-O σ-bonds to achieve cyclization, coupling and elimination reactions.
- Zhiguo Zhao
- , Yuanling Pang
- & Yao Wang
-
Article
| Open Access Asymmetric formal sp2-hydrocarbonations of dienes and alkynes via palladium hydride catalysis
Asymmetric formal sp2-hydrocarbonations of dienes and alkynes via palladium hydride catalysis
Sp2 carbon nucleophiles are generally not considered for transition metal-catalyzed asymmetric hydrofunctionalizations of unsaturated bonds due to challenges in cleaving corresponding inert sp2 C-H bonds. Here, the authors report a protocol to achieve asymmetric formal sp2 hydrocarbonations, including hydroalkenylation, hydroallenylation and hydroketenimination of both 1,3-dienes and alkynes via hydroalkylation and Wittig reaction cascade
- Ming-Qiao Tang
- , Zi-Jiang Yang
- & Zhi-Tao He
-
Article
| Open Access Metal-free photoinduced C(sp3)–H/C(sp3)–H cross-coupling to access α‑tertiary amino acid derivatives
Metal-free photoinduced C(sp3)–H/C(sp3)–H cross-coupling to access α‑tertiary amino acid derivatives
The cross-dehydrogenative coupling (CDC) reaction is the most efficient method for constructing α-tertiary amino acids (ATAAs). Here, the authors present a mild, metal-free CDC reaction for the construction of ATAAs with good regioselectivity.
- Yujun Li
- , Shaopeng Guo
- & Ke Zheng
-
Article
| Open Access Catalytic enantioselective reductive alkynylation of amides enables one-pot syntheses of pyrrolidine, piperidine and indolizidine alkaloids
Catalytic enantioselective reductive alkynylation of amides enables one-pot syntheses of pyrrolidine, piperidine and indolizidine alkaloids
Saturated α-alkyl aza-heterocycles are found in a wide array of bioactive molecules. Here, the authors disclosed a one-pot, catalytic enantioselective synthesis of pyrrolidine, piperidine and indolizidine alkaloids from amides and alkynes.
- Fang-Fang Xu
- , Jin-Quan Chen
- & Pei-Qiang Huang
-
Article
| Open Access 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
The phenyl ring is a basic structural element in chemistry. Here, the authors show the design, synthesis, and validation of 2-oxabicyclo[2.2.2]octane as a new saturated bioisostere with improved physicochemical properties
- Vadym V. Levterov
- , Yaroslav Panasiuk
- & Pavel K. Mykhailiuk
-
Article
| Open Access A metal-free photoactive nitrogen-doped carbon nanosolenoid with broad absorption in visible region for efficient photocatalysis
A metal-free photoactive nitrogen-doped carbon nanosolenoid with broad absorption in visible region for efficient photocatalysis
Carbon nanosolenoid materials with Riemann surfaces have unique structures and novel physical properties. Here, the authors report a nitrogen-doped carbon nanosolenoid heterojunction material with distinctive photocatalytic properties for light driven hydrogen production and organic transformations.
- Yu Zhou
- , Xinyu Zhang
- & Pingwu Du
-
Article
| Open Access The isocyanide SN2 reaction
The isocyanide SN2 reaction
The nucleophilic substitution reaction (SN2) is one of the oldest yet very useful organic transformations, but surprisingly, the nucleophilic character of isocyanides has never been explored in SN2 reactions. Here the authors show that isocyanides react as versatile nucleophiles in SN2 reactions with alkyl halides in a general manner to afford highly substituted secondary amides by in situ hydrolysis of the intermediate nitrilium ion.
- Pravin Patil
- , Qiang Zheng
- & Alexander Dömling
-
Article
| Open Access A biocatalytic platform for asymmetric alkylation of α-keto acids by mining and engineering of methyltransferases
A biocatalytic platform for asymmetric alkylation of α-keto acids by mining and engineering of methyltransferases
Catalytic asymmetric a-alkylation of carbonyl compounds represents a longstanding challenge in synthetic organic chemistry but general methods for the catalytic symmetric alkylation of carbonyl compounds remain elusive. Here, the authors mine and engineer methyltransferases as synthetically useful biocatalysts for the catalytic asymmetric alkylation reactions of carbonyl compounds.
- Shuyun Ju
- , Kaylee P. Kuzelka
- & Yang Yang
-
Article
| Open Access Synthesis of metalla-dual-azulenes with fluoride ion recognition properties
Synthesis of metalla-dual-azulenes with fluoride ion recognition properties
Azulene-based conjugated systems are of great interests due to their unusual structures and photophysical properties but incorporation of a transition metal into azulene skeleton presents remains elusive. Here, the authors describe an efficient [5 + 2] annulation reaction for the rapid construction of a metalla-dual-azulene.
- Hai-Cheng Liu
- , Kaidong Ruan
- & Haiping Xia
-
Article
| Open Access Stereospecific synthesis of silicon-stereogenic optically active silylboranes and general synthesis of chiral silyl Anions
Stereospecific synthesis of silicon-stereogenic optically active silylboranes and general synthesis of chiral silyl Anions
Silicon-stereogenic optically active silylboranes could potentially allow the formation of chiral silyl nucleophiles as well as the synthesis of various chiral silicon compounds but the synthesis of such silicon-stereogenic silylboranes remains underdeveloped. Here, the authors report the synthesis of silicon-stereogenic optically active silylboranes via a stereospecific Si–H borylation of chiral hydrosilanes in high yield enantiospecificity
- Xihong Wang
- , Chi Feng
- & Hajime Ito
-
Article
| Open Access A Bischler-Napieralski and homo-Mannich sequence enables diversified syntheses of sarpagine alkaloids and analogues
A Bischler-Napieralski and homo-Mannich sequence enables diversified syntheses of sarpagine alkaloids and analogues
The Mannich reaction is a well-established method for the synthesis of β-amino carbonyl compounds while the analogous reactions of homo-enol or its equivalents with imines or iminium ions are much less explored. Here, the authors describe a homo-Mannich reaction of cyclopropanol with imines generated via a Bischler-Napieralski reaction.
- Hanyue Qiu
- , Xinghai Fei
- & Min Zhang
-
Article
| Open Access All-round catalytic and atroposelective strategy via dynamic kinetic resolution for N-/2-/3-arylindoles
All-round catalytic and atroposelective strategy via dynamic kinetic resolution for N-/2-/3-arylindoles
While a variety of well-established methods enable the control of a stereogenic center, a catalytic method for controlling a stereogenic axis in one substrate is typically unavailable for controlling axial chirality in other substrates with a similar structure. Here, the authors report o-amidobiaryl as a flexible platform for chiral phosphoric acid catalyzed atroposelective dynamic kinetic resolution.
- Ahreum Kim
- , Chanhee Lee
- & Yongseok Kwon
-
Article
| Open Access Organophotocatalysed synthesis of 2-piperidinones in one step via [1 + 2 + 3] strategy
Organophotocatalysed synthesis of 2-piperidinones in one step via [1 + 2 + 3] strategy
The synthesis of 2-piperidinone derivatives remains challenging. Here, the authors develop an organophotocatalysed [1 + 2 + 3] strategy to enable the one-step access to diverse 2-piperidinones from ammonium salts, alkenes, and unsaturated carbonyl compounds.
- Yi-Dan Du
- , Shan Wang
- & Wei Shu
-
Article
| Open Access Cobalt-catalyzed atroposelective C−H activation/annulation to access N−N axially chiral frameworks
Cobalt-catalyzed atroposelective C−H activation/annulation to access N−N axially chiral frameworks
Atropisomers are an important chiral system which are widely present in natural products but atropisomerism bearing a N−N bond remains largely underdeveloped. Here, the authors report an efficient method for the enantioselective synthesis of N−N axially chiral frameworks under mild conditions via a cobalt-catalyzed atroposelective C-H activation/annulation
- Tong Li
- , Linlin Shi
- & Jun-Long Niu
-
Article
| Open Access Skeletal metalation of lactams through a carbonyl-to-nickel-exchange logic
Skeletal metalation of lactams through a carbonyl-to-nickel-exchange logic
Classical metalation reactions in organic synthesis typically involve metal-halogen exchange. Here, the authors introduce a metal-carbon exchange strategy that enables the skeletal editing of biologically relevant lactams by a Ni(0) compound.
- Hongyu Zhong
- , Dominic T. Egger
- & Bill Morandi
-
Article
| Open Access Kinetic resolution of substituted amido[2.2]paracyclophanes via asymmetric electrophilic amination
Kinetic resolution of substituted amido[2.2]paracyclophanes via asymmetric electrophilic amination
Planar chiral [2.2]paracyclophanes have found significant applications in various research fields. Here, Yang and coworkers report an efficient kinetic resolution method for various substituted amido[2.2]paracyclophanes via asymmetric C-H amination.
- Shaoze Yu
- , Hanyang Bao
- & Xiaoyu Yang
-
Article
| Open Access Synthesis, structural analysis, and properties of highly twisted alkenes 13,13’-bis(dibenzo[a,i]fluorenylidene) and its derivatives
Synthesis, structural analysis, and properties of highly twisted alkenes 13,13’-bis(dibenzo[a,i]fluorenylidene) and its derivatives
The rotation of a carbon double bond in an alkene can be efficiently accelerated by creating the high strain ground state and stabilizing the transition state of the process. Here, the authors report the synthesis, structures, and properties of several highly twisted alkenes.
- Hao-Wen Kang
- , Yu-Chiao Liu
- & Yao-Ting Wu
-
Article
| Open Access Catalytic asymmetric dearomatization of phenols via divergent intermolecular (3 + 2) and alkylation reactions
Catalytic asymmetric dearomatization of phenols via divergent intermolecular (3 + 2) and alkylation reactions
The catalytic asymmetric dearomatization (CADA) reaction is a powerful protocol for the assembly of three-dimensional cyclic compounds but phenols have been considered challenging substrates. Here, the authors report the chiral phosphoric acidcatalyzed divergent intermolecular CADA reactions of phenols with azoalkenes to obtain tetrahydroindolone and cyclohexadienone products in good yields with excellent ee values.
- Xiang Gao
- , Tian-Jiao Han
- & Guang-Jian Mei

 Catalyst control over pentavalent stereocentres
Catalyst control over pentavalent stereocentres