Abstract
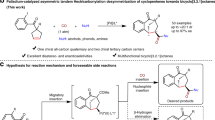
The catalytic asymmetric dearomatization (CADA) reaction has proved to be a powerful protocol for rapid assembly of valuable three-dimensional cyclic compounds from readily available planar aromatics. In contrast to the well-studied indoles and naphthols, phenols have been considered challenging substrates for intermolecular CADA reactions due to the combination of strong aromaticity and potential regioselectivity issue over the multiple nucleophilic sites (O, C2 as well as C4). Reported herein are the chiral phosphoric acid-catalyzed divergent intermolecular CADA reactions of common phenols with azoalkenes, which deliver the tetrahydroindolone and cyclohexadienone products bearing an all-carbon quaternary stereogenic center in good yields with excellent ee values. Notably, simply adjusting the reaction temperature leads to the chemo-divergent intermolecular (3 + 2) and alkylation dearomatization reactions. Moreover, the stereo-divergent synthesis of four possible stereoisomers in a kind has been achieved via changing the sequence of catalyst enantiomers.
Similar content being viewed by others
Introduction
The catalytic asymmetric dearomatization (CADA) reaction is a powerful transformation for directly converting planar aromatic compounds into enantioenriched three-dimensional cyclic scaffolds. Over the past decades, significant progress in this field has been made by You and others, which largely contributed to the facile synthesis of natural products and biologically important molecules1,2,3,4,5. For example, indole derivatives have been utilized frequently as model substrates for CADA reactions due to their relatively weak aromaticity6,7,8,9,10. Naphthols can be treated as conjugated enols in a sense, undergoing CADA transformations to prepare partially dearomatized naphthalenones11,12,13,14,15. On the other hand, the CADA reaction of phenols, readily available chemical feedstocks, still remains elusive (Fig. 1A). Oxidative asymmetric dearomatization of substituted phenols can be achieved by using oxidation systems as exampled by the chiral hypervalent iodine/m-CPBA16,17,18. Alternatively, the combination of oxidative dearomatization and subsequent asymmetric desymmetrization has been employed conventionally as a stepwise strategy for the asymmetric dearomatization of phenols (Fig. 1B)19,20,21. Direct non-oxidative asymmetric dearomatization of phenols is considered challenging, since the regioselectivity issue over the multiple nucleophilic sites (O, C2 as well as C4) is problematic. As such, phenol substrates are often delicately designed by incorporating an electrophilic group to conduct intramolecular dearomative cyclizations (Fig. 1C). For instance, metal-catalyzed intramolecular para-position allylation22,23, arylation24,25,26,27, and alkylation28 were disclosed by You, Tang and others. Recently, intramolecular ortho-position asymmetric dearomatization reactions of phenols have been reported by Ye and others via activation of ynamides or alkynes29,30. In a sharp contrast, intermolecular CADA reactions of common phenols, especially the cycloaddition reactions, are far less developed31.
Given the potential regio-, chemo-, and stereo-selectivity issues associated in the intermolecular CADA reaction of phenols, the rational selection of catalytic system and electrophile is key to success. Azoalkenes have emerged as versatile synthons in asymmetric synthesis, as they allow the rapid assembly of valuable N-heterocycles32,33. For instance, in situ generated azoalkenes have been employed as four-atom building blocks in copper-catalyzed (4 + n) reactions by Wang and others34,35,36,37,38,39. Recently, we have found that existent azoalkenes could act as three-atom synthons in chiral phosphoric acid (CPA)-catalyzed reacitons40,41,42,43. Along this line, we report herein the intermolecular CADA reaction of common phenols 1 with azoalkenes 2 under the catalysis of CPA (Fig. 1D). Notably, this protocol is featured by the temperature-dependent chemo-divergence and the sequence-dependent stereo-divergence44. By simply adjusting the reaction temperature, the chemo-divergent intermolecular (3 + 2) and alkylation dearomatization reactions of phenols have been accomplished, which deliver the corresponding products 3 and 4 bearing an all-carbon quaternary stereogenic center in good yields with excellent ee values. Meanwhile, with only a single CPA catalyst, changing the use sequence of catalyst enantiomers results in the stereo-divergent synthesis of four possible stereoisomers in a kind. Additionally, the constructed bicyclic tetrahydroindolone bearing an all-carbon quaternary stereogenic center is the core structure of some bioactive natural alkaloids, such as mesembrine, crinine and powelline45,46,47,48,49.
Results
We commenced our investigation with the model reaction between p-cresol 1a and azoalkene 2a (Table 1). In view of the potential regio- and chemo-selectivity issues, 3a–6a could be the products. Firstly, Brønsted acids were employed. Under the catalysis of CPA-1 in toluene at 25 °C, intermolecular CADA reaction occurred, delivering the only (3 + 2) product 3a in a good yield with a moderate enantioselectivity (entry 1). Then, a series of CPAs were screened to find the optimal catalyst. While all the binol-derived CPAs failed to improve the ee value (entries 2−6), spinol-derived CPA-7 gave an encouraging result wherein the ee value was increased to a relatively higher level (entry 7). So, CPA-7 was selected as the best catalyst for the subsequent condition screening. Solvent effect was studied, indicating that toluene was the best choice (entry 7 vs 8–10). Lowering reaction temperature contributed to the enantio-control (entries 11−12). Finally, additives were used to further optimize the reaction. With 5 Å molecular sieve, both the yield and ee value of product 3a could be improved to a high level (entry 14). Interestingly, Lewis acids led to a different regioselective result (see Supplementary Information for details). For example, while AgOTf offered the (3 + 2) dearomatization product 3a (entry 16), AgOAc resulted in the oxa-Michael addition product 5a (entry 15). We tried to control enantioselectivity of this oxa-Michael process by using silver chiral phosphate. However, only dearomative (3 + 2) product 3a was obtained (entry 17), which indicated silver chiral phosphate could also catalyze the dearomatization process. Notably, none of the C4-dearomatization alkylation product 4a and C2-Friedel-Crafts alkylation product 6a were observed during the optimization.
With the best reaction conditions in hand, the reaction generality of azoalkenes was examined. As shown in Fig. 2, the reaction was applicable to a variety of azoalkenes. The N-protective group could be varied from -CO2Bn (3a) to -CO2Me (3b), -CO2Et (3c), and -CO2fluorenylmethyl (3d) with consistently excellent ee values. The ester moiety appended to the C=C double bond had little effect on the reaction efficiency, all the tested substrates (3e−3j) were well-tolerated. In contrast, the R2 group significantly affected the enantioselectivity of the reaction. The larger the R2 group, the lower the ee values (3k−3l).Next, we turned our attention to the substrate scope of phenols (Fig. 2). Various phenols bearing alkyl substituents at 4-position, such as ethyl (3m), npropyl (3n), nbutyl (3o) and npentyl (3p) groups as well as allyl group (3q), served as suitable substrates for this CADA (3 + 2) reaction, allowing the installation of different moieties at the quaternary stereogenic center. In addition, the hindered cyclopropyl group (3r) worked equally well under standard conditions. Notably, the aryl group at the 4-position was also tolerated, as product 3s was readily prepared. Furthermore, we examined the tolerance of the reaction to di-substituted phenols. Products 3t−3z were prepared in good yields with good to excellent enantioselectivities under the modified conditions. Among them, the alkyl groups at the C2-position were in favor of enantio-control (3t−3v), while methoxy group resulted in a slight decrease in ee value (3w). Substituents at the C3-position had some delicate influence on the reaction. For example, 3x and 3y were obtained in good ee values, while 3z was formed in an excellent enantioselectivity. A regio-isomer was observed in the preparation of 3x (see Supplementary Information for details). Remarkably, the projected reaction was applicable to 1-naphthol, leading to the corresponding product 3a’ in good yield and ee value. The absolute configurations of the products were assigned based on X-ray crystallographic analysis of 3t.
Reaction conditions: 1 (0.2 mmol), 2 (0.24 mmol.), CPA-7 (10 mol%), and 5 Å MS (50 mg) in toluene (1 mL) at –30 °C for 12 h, isolated yields, ee and dr values were determined by chiral HPLC analysis, all dr >20:1, PG = NHCO2Bn. awith 3 Å MS (50 mg) and CPA-2 at −40 °C. bwith 3 Å MS (50 mg) and CPA-4 at 25 °C.
Encouraged by the success of CADA (3 + 2) reaction of mono- and di-substituted phenols, we then studied the substrate scop by employing tri-substituted phenols. Unexpectedly, the temperature-dependent chemo-divergent dearomatization reactions of phenol 1o were observed (Fig. 3A). At –30 °C, the standard conditions delivered the only dearomative alkylation product 4b in an excellent yield and with an excellent ee value. On the other hand, the CADA (3 + 2) reaction product 8 was obtained at 25 °C. Given the synthetic and biological importance of chiral cyclohexadienones, the substrate scope of this intermolecular catalytic asymmetric alkylation dearomatization had been studied (Fig. 3B). The examination of various azoalkenes indicated that the R2 group had some influence on the reaction efficiency. Reactions using methyl substituted azoalkenes (R2 = Me) were conducted smoothly, affording the corresponding cyclohexadienone products 4c–4i in good yields with excellent enantioselectivities (76–93% yields, 96–>99% ee). However, the steric hindered ethyl (4j), nbutyl (4k), and benzyl (4l) groups were not well compatible, which was same as the (3 + 2) process. The generality of tri-substituted phenols was also investigated. While electron-donating methoxy group (4m) afforded the consistently good yield and ee value, electron-withdrawing bromo group (4n) gave the result of no reaction.
To gain insights into the reaction mechanism, some control experiments were performed (Fig. 4). Firstly, enantioenriched 4b was subjected to the standard conditions at room temperature. Product 8 was formed in an excellent yield and with good stereoselectivities (Fig. 4A), which clearly suggested that 8 is a productive intermediate for the (3 + 2) reaction. More importantly, with the same enantioenriched 4b, (S)-CPA-7 led to a different diastereoisomer 9 (Fig. 4B), which implied the substrate control for the enantioselectivity and catalyst control for the diastereoselectivity. These results paved the way to a sequence-dependent stereo-divergent synthesis enabled by one chiral catalyst. Namely, changing the use sequence of (R)-CPA-7 and (S)-CPA-7 could result in the stereo-divergent synthesis of four possible stereoisomers in a kind. To demonstrate this concept, four possible stereoisomers of the (3 + 2) reaction products were prepared from 1o and 2a (Fig. 4E). However, under the catalysis of (±)-CPA-7, racemization of 4b was observed which led to a low ee value of the cyclizaiton product (Fig. 4C). On the other hand, when racemic 4b was employed, the enantio-convergent desymmetrization of cyclohexadienone was achieved (Fig. 4D), which will be fully investigated in the future. Based on these experimental results, a plausible reaction pathway and possible transition states (TS) were suggested in Fig. 4F. In TS-1, CPA-7 simultaneously activated phenol 1a and azoalkene 2a via dual hydrogen-bonding interactions. The CPA-catalyzed asymmetric dearomative alkylation of phenol 1a with azoalkene 2a occurred, generating the hydrazone intermediate Int-1 with a chiral carbon center. Subsequently, the group selectivity was accomplished via substrate-controlled enantioselective intramolecular cyclization. While pathway a led to the unfavored iminium Int-2 due to the sterically hindered cis-orientation of methyl and ester groups, pathway b gave the favored one Int-3. The final CPA-catalyzed diastereoselective proton transfer via tautomerization facilitated the (3 + 2) process and delivered the dearomative product 3a.
Finally, to examine the practicality of this protocol, a convenient gram-scale synthesis of 3u was conducted under standard reaction conditions (see Supplementary Information for details). Then a series of derivatizations were performed, further highlighting the synthetic utility (Fig. 5). Firstly, the enone motif could be manipulated via 1,2-addition, 1,4-hydrogenation and 1,2-reduction affording the corresponding product 10–12 in good yields and without erosion of enantiomeric purity. Besides, the protecting group on the azo nitrogen center could be easily removed, yielding product 13 in a good yield of 93%. Notably, N-allylic alkylation of 3a led to the formation of compound 14 incorporating a N−N axially stereogenic axis. The N−N bond cleavage had been achieved via a base-promoted eliminative strategy, furnishing the amine product 15.
Reaction conditions: i) 3c (0.2 mmol), CH3MgBr (0.3 mmol.), THF, 0 °C; ii) 3c (0.3 mmol), Pd/C, H2, MeOH, r.t.; iii) 3u (0.2 mmol), NaBH4 (0.2 mmol), MeOH, 0 °C; iv) 3 u (0.2 mmol), TFA (0.2 mL), CH2Cl2, 0 °C; v) 3a (0.2 mmol), ethyl 2-bromoacetate (0.24 mmol), Cs2CO3 (0.3 mmol), CH3CN, r.t.; vi) 3u (0.2 mmol), ethyl 2-bromoacetate (0.24 mmol), Cs2CO3 (0.3 mmol), CH3CN, r.t. then reflux.
Discussion
In conclusion, we have established the CPA-catalyzed divergent intermolecular CADA reactions of common phenols with azoalkenes, which deliver the tetrahydroindolone and cyclohexadienone products bearing an all-carbon quaternary stereogenic center in good yields with excellent ee values. As opposite to the previous dearomatization reactions involving indoles and naphthols, this work employs readily available phenols, which have been considered challenging substrates for intermolecular asymmetric dearomatization reactions due to the combination of strong aromaticity and the potential regioselectivity issue over the multiple nucleophilic sites (O, C2 as well as C4). Notably, this reaction is featured by the temperature-dependent chemo-divergence and the sequence-dependent stereo-divergence. Simply adjusting the reaction temperature leads to the chemo-divergent intermolecular (3 + 2) and alkylation dearomatization reactions. More importantly, stereo-divergent synthesis of four possible stereoisomers in a kind has been achieved by changing the sequence of catalyst enantiomers. Further applications of this protocol are currently ongoing in our laboratory and will be reported in due course.
Methods
Typical procedure for (3 + 2) reaction
Para substituted phenols 1 (0.20 mmol), 5 Å MS (50 mg), (R)-CPA-7 (10 mol%) were dissolved in toluene (1 mL), and azoalkenes 2 (0.24 mmol) were added dropwise at −30 °C. The reaction mixture was stirred at the same temperature for 12 h. The solvent was removed in vacuo and the crude product was separated by flash column chromatography on silica gel (petroleum ether/ethyl acetate 3:1–1:1) to afford the products 3.
Typical procedure for alkylation reaction
Tri-substituted phenols 1 (0.20 mmol), 3 Å MS (50 mg) and (R)-CPA-7 (10 mol%) were dissolved in toluene (1 mL), and azoalkenes 2 (0.24 mmol) were added dropwise at –30 °C. The reaction mixture was stirred for 12 h. The solvent was removed in vacuo and the crude product was separated by flash column chromatography on silica gel (petroleum ether/ethyl acetate 3:1–1:1) to afford the products 4.
Data availability
The authors declare that the data relating to the characterization of products, experimental protocols and the computational studies are available within the article and its Supplementary Information file. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers CCDC 2216396 for (–)–3t and CCDC 2203087 for (±)–3t. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Zhuo, C. X., Zhang, W. & You, S. L. Catalytic asymmetric dearomatization reactions. Angew. Chem. Int. Ed. 51, 12662–12686 (2012).
Zheng, C. & You, S.-L. Catalytic asymmetric dearomatization by transition-metal catalysis: a method for transformations of aromatic compounds. Chem 1, 830–857 (2016).
Cheng, Y.-Z., Zhang, X. & You, S.-L. Visible-light-mediated photocatalysis as a new tool for catalytic asymmetric dearomatization (CADA) reactions. Sci. Bull. 63, 809–811 (2018).
Zheng, C. & You, S. L. Advances in catalytic asymmetric dearomatization. ACS Cent. Sci. 7, 432–444 (2021).
Liu, Y.-Z., Song, H., Zheng, C. & You, S.-L. Cascade asymmetric dearomative cyclization reactions via transition-metal-catalysis. Nat. Synth. 1, 203–216 (2022).
Chen, J.-B. & Jia, Y.-X. Recent progress in transition-metal-catalyzed enantioselective indole functionalizations. Org. Biomol. Chem. 15, 3550–3567 (2017).
Huang, G. & Yin, B. Recent developments in transition metal-catalyzed dearomative cyclizations of indoles as dipolarophiles for the construction of indolines. Adv. Synth. Catal. 361, 405–425 (2019).
Saya, J. M., Ruijter, E. & Orru, R. V. A. Total synthesis of aspidosperma and strychnos alkaloids through indole dearomatization. Chem. Eur. J. 25, 8916–8935 (2019).
Zheng, C. & You, S. L. Catalytic asymmetric dearomatization (CADA) reaction-enabled total synthesis of indole-based natural products. Nat. Prod. Rep. 36, 1589–1605 (2019).
Sheng, F.-T., Wang, J.-Y., Tan, W., Zhang, Y.-C. & Shi, F. Progresses in organocatalytic asymmetric dearomatization reactions of indole derivatives. Org. Chem. Front. 7, 3967–3998 (2020).
Wu, W. T., Zhang, L. & You, S. L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 45, 1570–1580 (2016).
Sun, W., Li, G., Hong, L. & Wang, R. Asymmetric dearomatization of phenols. Org. Biomol. Chem. 14, 2164–2176 (2016).
An, J. Z. & Bandini, M. Recent advances in the catalytic dearomatization of naphthols. Eur. J. Org. Chem. 2020, 4087–4097 (2020).
Shen, D., Chen, Q., Yan, P., Zeng, X. & Zhong, G. Enantioselective dearomatization of naphthol derivatives with allylic alcohols by cooperative iridium and bronsted acid catalysis. Angew. Chem. Int. Ed. 56, 3242–3246 (2017).
Xia, Z. L., Zheng, C., Xu, R. Q. & You, S. L. Chiral phosphoric acid catalyzed aminative dearomatization of alpha-naphthols/Michael addition sequence. Nat. Commun. 10, 3150 (2019).
Uyanik, M., Yasui, T. & Ishihara, K. Hydrogen bonding and alcohol effects in asymmetric hypervalent iodine catalysis: enantioselective oxidative dearomatization of phenols. Angew. Chem. Int. Ed. 52, 9215–9218 (2013).
Baker Dockrey, S. A., Lukowski, A. L., Becker, M. R. & Narayan, A. R. H. Biocatalytic site- and enantioselective oxidative dearomatization of phenols. Nat. Chem. 10, 119–125 (2018).
Hashimoto, T., Shimazaki, Y., Omatsu, Y. & Maruoka, K. Indanol-based chiral organoiodine catalysts for enantioselective hydrative dearomatization. Angew. Chem. Int. Ed. 57, 7200–7204 (2018).
Gu, Q. in Asymmetric Dearomatization Reactions 103–128, https://doi.org/10.1002/9783527698479.ch5 (Wiley-VCH Verlag GmbH & Co. KGaA, 2016).
Chen, B., He, C.-Y., Chu, W.-D. & Liu, Q.-Z. Recent advances in the asymmetric transformations of achiral cyclohexadienones. Org. Chem. Front. 8, 825–843 (2021).
Shu, T. & Cossy, J. Asymmetric desymmetrization of alkene-, alkyne- and allene-tethered cyclohexadienones using transition metal catalysis. Chem. Soc. Rev. 50, 658–666 (2021).
Wu, Q. F. et al. Iridium-catalyzed intramolecular asymmetric allylic dearomatization of phenols. Angew. Chem. Int. Ed. 50, 4455–4458 (2011).
Wang, Y. et al. Enantioselective desymmetrization of bisphenol derivatives via Ir-catalyzed allylic dearomatization. J. Am. Chem. Soc. 142, 19354–19359 (2020).
Rousseaux, S., Garcia-Fortanet, J., Del Aguila Sanchez, M. A. & Buchwald, S. L. Palladium(0)-catalyzed arylative dearomatization of phenols. J. Am. Chem. Soc. 133, 9282–9285 (2011).
Xu, R. Q., Gu, Q., Wu, W. T., Zhao, Z. A. & You, S. L. Construction of erythrinane skeleton via Pd(0)-catalyzed intramolecular dearomatization of para-aminophenols. J. Am. Chem. Soc. 136, 15469–15472 (2014).
Du, K. et al. Enantioselective palladium-catalyzed dearomative cyclization for the efficient synthesis of terpenes and steroids. Angew. Chem. Int. Ed. 54, 3033–3037 (2015).
Xu, R. Q., Yang, P., Zheng, C. & You, S. L. Pd‐catalyzed asymmetric intramolecular arylative dearomatization of para‐aminophenols. Chin. J. Chem. 38, 683–689 (2020).
Nakayama, H., Harada, S., Kono, M. & Nemoto, T. Chemoselective asymmetric intramolecular dearomatization of phenols with alpha-diazoacetamides catalyzed by silver phosphate. J. Am. Chem. Soc. 139, 10188–10191 (2017).
Zhang, Y. Q. et al. Asymmetric dearomatization catalysed by chiral Bronsted acids via activation of ynamides. Nat. Chem. 13, 1093–1100 (2021).
Zhao, K. et al. Enantioselective dearomative cyclization enabled by asymmetric cooperative gold catalysis. Angew. Chem. Int. Ed. 61, e202207518 (2022).
Shao, L. & Hu, X. P. Copper-catalyzed intermolecular asymmetric propargylic dearomatization of phenol derivatives. Chem. Commun. 53, 8192–8195 (2017).
Lopes, S. M. M., Cardoso, A. L., Lemos, A. & Pinho E Melo, T. M. V. D. Recent advances in the chemistry of conjugated nitrosoalkenes and azoalkenes. Chem. Rev. 118, 11324–11352 (2018).
Wei, L., Shen, C., Hu, Y. Z., Tao, H. Y. & Wang, C. J. Enantioselective synthesis of multi-nitrogen-containing heterocycles using azoalkenes as key intermediates. Chem. Commun. 55, 6672–6684 (2019).
Chen, J. R. et al. Enantioselective synthesis of dihydropyrazoles by formal [4+1] cycloaddition of in situ-derived azoalkenes and sulfur ylides. J. Am. Chem. Soc. 134, 6924–6927 (2012).
Tong, M. C. et al. Catalytic asymmetric synthesis of [2,3]-fused indoline heterocycles through inverse-electron-demand aza-Diels-Alder reaction of indoles with azoalkenes. Angew. Chem. Int. Ed. 53, 4680–4684 (2014).
Huang, R., Chang, X., Li, J. & Wang, C. J. Cu(I)-catalyzed asymmetric multicomponent cascade inverse electron-demand Aza-Diels-Alder/nucleophilic addition/ring-opening reaction involving 2-methoxyfurans as efficient dienophiles. J. Am. Chem. Soc. 138, 3998–4001 (2016).
Wei, L. et al. Copper(I)-catalyzed asymmetric 1,3-dipolar [3+4] cycloaddition of nitrones with azoalkenes. Adv. Synth. Catal. 358, 3748–3752 (2016).
Wei, L., Zhu, Q., Song, Z. M., Liu, K. & Wang, C. J. Catalytic asymmetric inverse electron demand Diels-Alder reaction of fulvenes with azoalkenes. Chem. Commun. 54, 2506–2509 (2018).
Bai, L. et al. Catalytic asymmetric [4+1] spiroannulation of α-bromo-β-naphthols with azoalkenes by an electrophilic dearomatization/S RN 1-debromination approach. CCS Chemistry 4, 1054–1064 (2022).
Mei, G. J., Tang, X., Tasdan, Y. & Lu, Y. Enantioselective dearomatization of indoles by an azoalkene-enabled (3+2) reaction: access to pyrroloindolines. Angew. Chem. Int. Ed. 59, 648–652 (2020).
Mei, G.-J. et al. Catalytic asymmetric formal [3+2] cycloaddition of azoalkenes with 3-vinylindoles: synthesis of 2,3-dihydropyrroles. iScience 23, 100873 (2020).
Mei, G.-J. et al. Chemo-, regio- and enantioselective dearomative (3+2) reaction of non-functionalized 1-naphthols. Chem Catal. 2, 386–399 (2022).
Han, T. J., Zhang, Z. X., Wang, M. C., Xu, L. P. & Mei, G. J. The rational design and atroposelective synthesis of axially chiral C2-arylpyrrole-derived amino alcohols. Angew. Chem. Int. Ed. 61, e202207517 (2022).
Liu, X. J. et al. Sequence-dependent stereodivergent allylic alkylation/fluorination of acyclic ketones. Angew. Chem. Int. Ed. 59, 2039–2043 (2020).
Smith, E., Hosansky, N., Shamma, M. & Moss, J. B. Mesembrine enol ether and the absolute configuration of mesembrine. Chem. Ind. 402–403 (1961).
Kobayashi, S. et al. Alkaloidal constituents of Crinum latifolium and Crinum bulbispermum (Amaryllidaceae). Chem. Pharm. Bull. 32, 3015–3022 (1984).
Abdel-Halim, O. B., Morikawa, T., Ando, S., Matsuda, H. & Yoshikawa, M. New crinine-type alkaloids with inhibitory effect on induction of inducible nitric oxide synthase from Crinum yemense. J. Nat. Prod. 67, 1119–1124 (2004).
Chuang, K. V., Navarro, R. & Reisman, S. E. Short, enantioselective total syntheses of (-)-8-demethoxyrunanine and (-)-cepharatines A, C, and D. Angew. Chem. Int. Ed. 50, 9447–9451 (2011).
Herzon, S. B., Calandra, N. A. & King, S. M. Efficient entry to the hasubanan alkaloids: first enantioselective total syntheses of (-)-hasubanonine, (-)-runanine, (-)-delavayine, and (+)-periglaucine B. Angew. Chem. Int. Ed. 50, 8863–8866 (2011).
Acknowledgements
We are grateful for the generous financial support from Natural Science Foundation of Henan Province (222300420084 to G.-J.M.), application research plan of Key Scientific Research Projects in Colleges and Universities of Henan Province (22A150056 to G.-J.M.), and National Natural Science Foundation of China (22208302 to X.X.).
Author information
Authors and Affiliations
Contributions
X.G. and T.-J.H. performed and analyzed the experiments. B.-B.L., Y.-Z.H., S.-K.J., X.X. and M.-C.W. participated in the early development of the project. X.-X.H. and D.W. did the calculations. G.-J.M. conceived and designed the project. G.-J.M. overall supervised the project. All authors prepared this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Zhihui Shao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gao, X., Han, TJ., Li, BB. et al. Catalytic asymmetric dearomatization of phenols via divergent intermolecular (3 + 2) and alkylation reactions. Nat Commun 14, 5189 (2023). https://doi.org/10.1038/s41467-023-40891-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-40891-w
This article is cited by
-
Reaction strategies for the meta-selective functionalization of pyridine through dearomatization
Molecular Diversity (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.