Abstract
Although aryl triflates are essential building blocks in organic synthesis, the applications as aryl radical precursors are limited. Herein, we report an organomediated electrochemical strategy for the generation of aryl radicals from aryl triflates, providing a useful method for the synthesis of aryl sulfonyl fluorides from feedstock phenol derivatives under very mild conditions. Mechanistic studies indicate that key to success is to use catalytic amounts of 9, 10-dicyanoanthracene as an organic mediator, enabling to selectively active aryl triflates to form aryl radicals via orbital-symmetry-matching electron transfer, realizing the anticipated C–O bond cleavage by overcoming the competitive S–O bond cleavage. The transition-metal-catalyst-free protocol shows good functional group tolerance, and may overcome the shortages of known methods for aryl sulfonyl fluoride synthesis. Furthermore, this method has been used for the modification and formal synthesis of bioactive molecules or tetraphenylethylene (TPE) derivative with improved quantum yield of fluorescence.
Similar content being viewed by others
Introduction
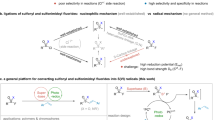
Phenols and its derivatives are ubiquitous in many natural products and bioactive molecules. They can be obtained from industrial Hock process or abundant and renewable biomass such as lignin1,2. Therefore, developing transformations based on inexpensive phenols and their derivatives is meaningful3,4. Because of the inertness of C–O bonds in phenols, the introduction of an electron-withdrawing group to oxygen is a common activation method. For example, as a typical kind of phenol derivatives, aryl sulfonates5,6 can serve as electrophiles, participating in versatile transition metal-catalyzed cross-coupling reactions via the oxidation addition of metal catalyst with C–O bonds (two-electron pathway) (Fig. 1a)7,8,9,10,11,12. Besides, recently, excellent examples by using ultraviolet light (254 nm) or the excited state of Pd(0) to promote the formation of very reactive aryl radicals from aryl sulfonates via single electron pathway have been disclosed by Li13,14 and Gevorgyan15, respectively. These new activation strategies by connecting the applications of aryl sulfonates with the versatile aryl radical chemistry16,17 undoubtedly broaden the synthetic scope of phenol derivatives18,19,20. Expanding the potential of aryl sulfonates with a benign activation model for new reaction design is appealing and remains underdeveloped.
On the other hand, driven by the intrinsic advantage of electrosynthesis where an electron could serve as a green reagent to active substrate21,22,23,24,25,26,27,28 and our work in electrosynthesis29,30,31,32,33, we envisioned that the reduction of aryl sulfonates at cathode might lead to the generation of aryl radical, similar to aryl halides34,35,36,37. Furthermore, such an electrochemical activation strategy enables to design of new transformations by employing the advantage of electrosynthesis. However, it is nontrivial to realize such a naive hypothesis as aryl sulfonates have shown different reactivity towards electrolysis than that of aryl halides. This can be attributed to the fact that aryl sulfonates have two different electron-deficient sites (aryl and S atom), and the sulfur atom in aryl sulfonates is the much more electron-deficient one (NBO charge, Fig. 1). Furthermore, upon reduction by chemical reducing reagents38,39 or direct electrolysis conditions40,41, several precedent examples have identified that the formation of phenol salts via selective transferring electron to S to cleave S–O bond (Fig. 1a). Therefore, one prerequisite to form aryl radicals from aryl sulfonates is to find a suitable condition which enables to selectively induce the SET process by tuning the transfer of electron from more electron-deficient sulfur atom to the aryl ring. Herein, inspired by recent electrochemical examples which utilize organomediator as an effective electron-transfer mediator42,43,44,45,46,47,48,49,50,51,52,53,54,55 to activate substrates, we envisioned that by using an orbital-symmetry-matching strategy, whereas electron transfer from the HOMO orbital of a π-ring structure in electron-rich catalytic species to the electron-deficient LUMO orbital of aryl moiety in aryl sulfonates might achieve such selectivity. Indeed, after extensive studies, we found that simple 9,10-dicyanoanthracene could serve as an efficient electron-transfer mediator for the purpose, leading to the formation of key aryl radicals after fragmentation under mild electrochemical conditions. Furthermore, such a strategy enables to achieve the fluorosulfonylation under transition-metal-free conditions with broad functional group tolerance by harnessing the merits of paired electrolysis (Fig. 1b). To the best of our knowledge, although several methods have been reported to prepare the aryl sulfonyl fluorides56,57,58,59,60,61,62,63,64,65,66, protocols from simple phenol derivatives with broad functional group tolerance has yet to be disclosed67. As pointed out by Willis, the use of aryl triflates to realize the fluorosulfonylation via Pd catalysis has failed68. Herein, we wish to wish to report our preliminary results.
Results
To optimize the suitable conditions for the designed aryl radical formation from aryl sulfonates by electrochemical method, simple p-tolyl trifluoromethanesulfonate 1a (Ered = −1.0 V vs. Ag/AgCl) was used as the model substrate, and the fluorosulfonylation had been designed for evaluating the efficiency. As shown in Table 1, the desired fluorosulfonylation could work smoothly, delivering the anticipated 3a with 75% yield (Faraday efficiency is 74%) under the optimal conditions (20 mol% 9,10-dicyanoanthracene M-1 as the key organomediator) by overcoming the formation of p-cresol 3b via S–O bond cleavage (entry 1). Noteworthy, a trace amount of 4,4’-dimethyl-1,1’-biphenyl 3c has also been detected by GC–MS analysis, implying the involvement of 4-methylphenyl radical in this case. During the process, the voltage ranges from 3.49 to 3.1 V, corresponding to a cathodic potential of around −2.4 V vs. Ag/AgCl, and the following five points should be highlighted. (1) As designed, the organomediator M-1 is essential for the transformation, as the use of other fused polycyclic aromatic compounds or ketones (M-2–M-5) led to diminished yields along with the observation of sustainable amounts of p-cresol 3a’ (entries 2–6). Furthermore, the reactivity is closely associated with the reduction potential of these organomediators, as the formation of p-cresol as the major product via S–O bond cleavage was observed in the presence of mediators with a reduction potential less than that of 1a. As a comparison, no 3a was formed in the absence of organomediator (entry 7). (2) To match the rate of aryl radical formation with the subsequent fluorosulfonylation, 20 mol% loading is optimal (entries 8 and 9). (3) The screen of other electrochemical parameters (electrolyte, electrode, and electric current density) as well as fluoride source implies the essential role of these for good reactivity (entries 10–12). In addition, reducing or increasing the current resulted in lower efficiency (entry 13). (4) Electricity was proven to be essential as no reaction took place in its absence (entry 14). (5) In comparison, no reaction took place by using 4-MeC6H4OMe, 4-MeC6H4OAc, 4-MeC6H4OPO(OMe)2, 4-MeC6H4Br, or 4-MeC6H4I (the Ered of these compounds is −2.6, −2.5, −3.0, −2.6 and −2.5 V, vs. Ag/AgCl, respectively) as aryl source (entry 15).
Having established the optimal condition for the desired fluorosulfonylation, the scope with versatile phenol-derived trifluoromethanesulfonates 1 was carefully evaluated. It turns out that our new strategy shows good functional group tolerance. As shown in Fig. 2, a variety of functional groups such as halides (F, Cl, Br, I), methoxyl, amide, ketone carbonyl, ester, cyano, and sulfonyl were all compatible, giving rise to products 3a–3ac with moderate to good yields (43–81%). In addition, the introduction of substituents at ortho- position has little effect on the yield (3ad–3ai). Naphthalene, pyrrole, or quinoline derivatives are suitable, and these products 3aj–3am were isolated with 59–76% yields. Our method can be used for the fluorosulfonylation of natural products or drug derivatives with 55–76% yields (4a–4g). The compatibility with amide, ester, ketone, or thienyl groups highlights the advantage of our protocol.
For details, please see Supplementary Information (SI).
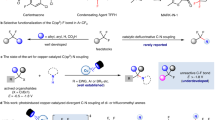
To demonstrate the synthetic applications, we have conducted the following experiments. (1) The model reaction can be facilely conducted in one pot. As shown in Fig. 3a, the desired 3a could be isolated with 72% from 4-methylphenol after two steps. (2) Our model reaction could be conducted at 10 mmol scale, indicating the practicability of the process (Fig. 3b). (3) In addition, product 3ae can react with tetrahydropyrrole to form sulfonamide 5, a key intermediate for the preparation of selective ƙ opioid antagonist 669 (Fig. 3c). (4) The selective fluorosulfonylation from 4-(1,2,2-triphenylvinyl)phenyl trifluoromethanesulfonate 7 enables to deliver 8. More essentially, by using the good electron-withdrawing ability of the FSO2 group, the emission peak of 8 was redshifted to 467 nm from 453 nm of 7, accompanied by an increase of the quantum yield of fluorescence from 3.3% to 36.0% (Fig. 3d).
To shed light on the mechanism, we conducted the following experiments. (1) First, the use of radical scavengers such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) or ethene-1,1-diyldibenzene 9 enables to detect the two radical-adducts 11 and 12 by high-resolution mass spectrometry (HRMS), respectively (Fig. 4a). These, together with the detection of 4,4’-dimethyl-1,1’-biphenyl in our model reaction (Table 1), imply the possible involvement of aryl sulfonyl and aryl radicals for our transformation. (2) Second, the model reaction was tested in a divided cell, and no 3a was detected (Fig. 4b), indicating the paired electrosynthesis nature of the reaction. (3) In addition, cyclic voltammetry (CV) studies were conducted to further understand the role of organomediator M-1. As shown in Fig. 5, the reduction peak of 1a and M-1 at −1.0 and −0.95 V vs. Ag/AgCl could be observed (for the full CV and detailed discussions about 1a, please see part 8, Supplementary Information), respectively. Along with the addition of excess amounts of 1a, a decrease in oxidation current and an increase in reduction current of M-1 were detected. (4) To further elucidate the key role of M-1 in mediating the electrochemical reactivity of ArOTf, exhaustive electrolysis experiments have been conducted. As shown in Fig. 4c, while direct electrolysis of 1a at a constant potential of 1.0 V only led to 4-methylphenol 3b, the use of 2.0 and 3.0 V led to the observation of 4-methylphenol 3b and toluene, too. The formation of both 3b and toluene indicates the two different electrochemical pathways for 1a. Nevertheless, after the addition of M-1 (20 mol%), 4-methylphenol 3a’ has been inhibited, with the formation of 4,4’-dimethyl-1,1’-biphenyl 3a’ as the main product (Fig. 4d). These data implied that M-1 could serve as a single-electron transfer mediator for the selective reduction of aryl triflates. The formation of 3a” with 63% isolated yield also provides a transition-metal-free electrochemical protocol for achieving the reduction cross-coupling between two aryl triflates40.
Experiments were conducted by using glass carbon as the working electrode, Pt plate, and Ag/Ag+ as the counter and reference electrode. Scan rate: 100 mV/s. Solvent: MeCN/nBu4NClO4 (0.1 M). a Background, b M-1 (25.0 mM), c 1a (25.0 mM), d and e CVs of M-1 performed in the presence of increasing equivalents of 1a.
Based on the experimental results and literature reports, two plausible mechanisms are proposed for such fluorosulfonylation (using 1a as the model substrate, Fig. 6). At first, M-1 was reduced to a radical anion I at the cathode. After that, intermediate I could selectively reduce 1a to form the key 4-methylphenyl radical II. The subsequent trap by SO2 obtains species III. As postulated by Tlili70, DABCO will be oxidized to radical cation IV at the anode for path 1. The interaction of III and IV delivers the species V. The subsequent nucleophilic substitution with F− gives rise to the observed 3a. On the other hand, as proposed by Cheng and Zhou61, III could be oxidized to form sulfonyl cation VI, and the nucleophilic addition with F− to form 3a is also possible. Control experiment by using a divided cell led to no 3a (Fig. 4b) also supports these hypotheses.
To give a deep understanding about the selective electron transfer step, additional DFT calculations were conducted. As shown in Fig. 7a, the highest occupied molecular orbital (HOMO) of M-1·‒ is mainly composed of the π orbital of the anthracene, whose energy level is close to the one of the lowest unoccupied molecular orbital (LUMO) of 1b, composed by the π* of the phenyl ring. Charge transfer (CT) takes place smoothly between the symmetry-matched orbitals from M-1·‒ to 1b, promoting the formation of adduct A (Fig. 7a). After this, the release of M-1 from adduct I result in radical anion 1b·‒. DFT calculation indicates the need for 9.2 kcal/mol energy for this step (Fig. 7b). Eventually, the facile fragmentation by releasing OTf− group from 1b·‒ delivers the key phenyl radical II, which will be trapped by SO2 to deliver radical III in a dramatic exothermic way (for details, please see SI).
Discussion
In summary, by using 9, 10-dicyanoanthracene as an effective organomediator, the selective reduction of aryl triflates to aryl radicals via orbital-symmetry-matching electron transfer has been disclosed, so as to realize the anticipated C–O bond cleavage by overcoming the competitive S–O bond cleavage. This enables the development of a new and practical method for the synthesis of aryl sulfonyl fluorides from feedstock phenol derivatives under mild electrochemical conditions. The synthetic applications of such fluorosulfonylation from phenol derivatives have been explored. Further applications of such strategy for other new reactions are ongoing in our laboratories.
Methods
General procedure for the fluorosulfonylation of 1
To the cell was added aryl triflate 1 (0.2 mmol), DABSO (36 mg, 0.15 mmol), KHF2 (46.8 mg, 0.6 mmol), n-Bu4NClO4 (0.05 M, 68.2 mg), 9, 10-dicyanoanthracene (M-1, 9.1 mg, 0.04 mmol), CH3CN (4 mL). The tube was installed with a Pt plate (1.0 × 1.0 cm2) as the cathode and reticulated vitreous carbon (RVC) (1.0 × 1.0 cm2) as the anode. The mixture was electrolyzed using 12 mA at room temperature under magnetic stirring. The reaction mixture was poured into ethyl acetate (40 mL), washed with water two times (10 mL × 2), dried over Na2SO4, and concentrated in vacuo. The residue was purified by column chromatography on silica gel using a mixture of petroleum ether/EtOAc as eluent to afford the desired pure product 3, 4, or 8.
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information file. All other data are available from the corresponding author Xianqiang Kong or Zhong-Yan Cao.
References
Weber, M. & Weber, M. M. Kleine-Boymann in Phenol in Ullmann’s Encyclopedia of Industrial Chemistry Vol. 26 513–519 (Wiley-VCH, Weinheim, 2004).
Gardziella, A., Pilato, L. A. & Knop, A. Phenolic Resins: Chemistry, Applications, Standardization, Safety and Ecology (Springer Science & Business Media, 2013).
Wu, W.-T., Zhang, L. & You, S.-L. Catalytic asymmetric dearomatization (CADA) reactions of phenol and aniline derivatives. Chem. Soc. Rev. 45, 1570–1580 (2016).
Qiu, Z. & Li, C.-J. Transformations of less-activated phenols and phenol derivatives via C–O cleavage. Chem. Rev. 120, 10454–10515 (2020).
Ritter, K. Synthetic transformations of vinyl and aryl triflates. Synthesis 1993, 735–762 (1993).
Chassaing, S., Specklin, S., Weibel, J.-M. & Pale, P. Vinyl and aryl sulfonates: Preparations and applications in total synthesis. Curr. Organ. Synth. 9, 806–827 (2012).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Echavarren, A. M. & Stille, J. K. Palladium-catalyzed cross-coupling reactions of organoboron compounds. J. Am. Chem. Soc. 109, 5478–5486 (1987).
Hartwig, J. F. Transition metal catalyzed synthesis of arylamines and aryl ethers from aryl halides and triflates: Scope and mechanism. Angew. Chem. Int. Ed. 37, 2046–2067 (1998).
Yang, B. H. & Buchwald, S. L. Palladium-catalyzed amination of aryl halides and sulfonates. J. Organomet. Chem. 576, 125–146 (1999).
Zhang, Y.-F. & Shi, Z.-J. Exploration of new C−O electrophiles in cross-coupling reactions. Acc. Chem. Res. 43, 1486–1495 (2010).
Zarate, C., van Gemmeren, M., Somerville, R. J., & Martin, R. Phenol derivatives: modern electrophiles in cross-coupling reactions. In Advances in Organometallic Chemistry (ed P. Pérez, J.) 143−222 (Academic Press, 2016).
Liu, W., Yang, X., Gao, Y. & Li, C.-J. Simple and efficient generation of aryl radicals from aryl triflates: synthesis of aryl boronates and aryl iodides at room temperature. J. Am. Chem. Soc. 139, 8621–8627 (2017).
Dou, Q., Geng, L., Cheng, B., Li, C.-J. & Zeng, H. Photoinduced transition-metal and external photosensitizer free cross-coupling of aryl triflates with trialkyl phosphites. Chem. Commun. 57, 8429–8432 (2021).
Ratushnyy, M., Kvasovs, N., Sarkar, S. & Gevorgyan, V. Visible-light-induced palladium-catalyzed generation of aryl radicals from aryl triflates. Angew. Chem. Int. Ed. 59, 10316–10320 (2020).
Kvasovs, N. & Gevorgyan, V. Contemporary methods for generation of aryl radicals. Chem. Soc. Rev. 50, 2244–2259 (2021).
Wu, X., Ma, Z., Feng, T. & Zhu, C. Radical-mediated rearrangements: past, present, and future. Chem. Soc. Rev. 50, 11577–11613 (2021).
Shaikh, R. S., Düsel, S. J. S. & Kӧnig, B. Visible-light photo-Arbuzov reaction of aryl bromides and trialkyl phosphites yielding aryl phosphonates. ACS Catal. 6, 8410–8414 (2016).
Jin, S. et al. Visible light-induced borylation of C–O, C–N, and C–X bonds. J. Am. Chem. Soc. 142, 1603–1613 (2020).
Raviola, C., Canevari, V., Protti, S., Albini, A. & Fagnoni, M. Metal-free arylations via photochemical activation of the Ar–OSO2R bond in aryl nonaflates. Green. Chem. 15, 2704–2708 (2013).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Tang, S., Liu, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem. 4, 27–45 (2018).
Xiong, P. & Xu, H.-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 52, 3339–3350 (2019).
Ackermann, L. Metalla-electrocatalyzed C–H activation by earth-abundant 3d metals and beyond. Acc. Chem. Res. 53, 84–104 (2020).
Siu, J. C., Fu, N. K. & Lin, S. Catalyzing electrosynthesis: a homogeneous electrocatalytic approach to reaction discovery. Acc. Chem. Res. 53, 547–560 (2020).
Yu, Y., Guo, P., Zhong, J.-S., Yuan, Y. & Ye, K.-Y. Merging photochemistry with electrochemistry in organic synthesis. Org. Chem. Front. 7, 131–135 (2020).
Ma, C. et al. Recent advances in organic electrosynthesis employing transition metal complexes as electrocatalysts. Sci. Bull. 66, 2412–2429 (2021).
Xu, C. et al. Recent applications of homogeneous catalysis in electrochemical organic synthesis. CCS Chem. 4, 1120–1152 (2022).
Kong, X., Liu, Y., Lin, L., Chen, Q. & Xu, B. Electrochemical synthesis of enaminones via a decarboxylative coupling reaction. Green Chem. 21, 3796–3801 (2019).
Kong, X. et al. Cyanation and cyanomethylation of trimethylammonium salts via electrochemical cleavage of C–N bonds. Org. Chem. Front. 9, 1288–1294 (2022).
Kong, X. et al. A practically unified electrochemical strategy for Ni-catalyzed decarboxylative cross-coupling of aryl trimethylammonium salts. Org. Lett. 24, 2137–2142 (2022).
Kong, X., Chen, X., Chen, Y. & Cao, Z.-Y. Scalable electrocatalytic intermolecular acylcyanation and aminocyanation of alkenes. J. Org. Chem. 87, 7013–7021 (2022).
Kong, X. et al. Selective fluorosulfonylation of thianthrenium salts enabled by electrochemistry. Org. Lett. 25, 581–586 (2023).
Grimshaw, J. Reduction Bond Cleavage Processes-I in Electrochemical Reactions and Mechanisms in Organic Chemistry 89–157 (Elsevier Science, Amsterdam, 2000).
Fuchigami, T., Kasuga, M. & Konno, A. Electro-organic chemistry of fullerenes. Part 1. Indirect cathodic reduction of vic-dihalides and perfluoroalkyl halides using C60 as mediator. Cyclic voltammetric study and preparative-scale electrolysis. J. Electroanal. Chem. 411, 115–119 (1996).
Folgueiras-Amador, A. A. et al. Cathodic radical cyclisation of aryl halides using a strongly-reducing catalytic mediator in flow. Angew. Chem. Int. Ed. 61, e202203694 (2022).
Wan, Y. et al. Metal-free electrochemical carboxylation of organic halides in the presence of catalytic amounts of an organomediator. Angew. Chem. Int. Ed. 61, e202210201 (2022).
Jolly, P. I. et al. Reactions of triflate esters and triflamides with an organic neutral super-electron-donor. Org. Biomol. Chem. 10, 5807–5810 (2012).
Radivoy, G., Alonso, F. & Yus, M. Reduction of sulfonates and aromatic compounds with the NiCl2·2H2O Li-arene (cat.) combination. Tetrahedron 55, 14479–14490 (1999).
Jutand, A., Negri, S. & Mosleh, A. Palladium catalysed electrosynthesis using aryl trifluoromethanesulfonates (triflates). Synthesis of biaryls and aromatic carboxylic acids. J. Chem. Soc. Chem. Commun. 1729–1730 (1992).
Civitello, E. R. & Rapoport, H. The regioselective cleavage of aryl tosylates by electrochemical reduction. J. Org. Chem. 57, 834–840 (1992).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: Basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Wang, F. & Stahl, S. S. Electrochemical oxidation of organic molecules at lower overpotential: accessing broader functional group compatibility with electron−proton transfer mediators. Acc. Chem. Res. 53, 561–574 (2020).
Badalyan, A. & Stahl, S. S. Cooperative electrocatalytic alcohol oxidation with electron–proton-transfer mediators. Nature 535, 406–410 (2016).
Xiong, P., Xu, H.-H., Song, J. & Xu, H.-C. Electrochemical difluoromethylarylation of alkynes. J. Am. Chem. Soc. 140, 2460–2464 (2018).
Zhang, W., Carpenter, K. L. & Lin, S. Electrochemistry broadens the scope of flavin photocatalysis: photoelectrocatalytic oxidation of unactivated alcohols. Angew. Chem. Int. Ed. 59, 409–417 (2020).
Niu, L. et al. Manganese-catalyzed oxidative azidation of C(sp3)–H bonds under electrophotocatalytic conditions. J. Am. Chem. Soc. 142, 17693–17702 (2020).
Galvin, C. M. & Waymouth, R. M. Electron-rich phenoxyl mediators improve thermodynamic performance of electrocatalytic alcohol oxidation with an iridium pincer complex. J. Am. Chem. Soc. 142, 19368–19378 (2020).
Huang, H. & Lambert, T. H. Electrophotocatalytic acetoxyhydroxylation of aryl olefins. J. Am. Chem. Soc. 143, 7247–7252 (2021).
Saito, M. et al. N-Ammonium ylide mediators for electrochemical C–H oxidation. J. Am. Chem. Soc. 143, 7859–7867 (2021).
Shen, T. & Lambert, T. H. Electrophotocatalytic diamination of vicinal C–H bonds. Science 371, 620–626.
Wang, Z.-H. et al. TEMPO-enabled electrochemical enantioselective oxidative coupling of secondary acyclic amines with ketones. J. Am. Chem. Soc. 143, 15599–15605 (2021).
Liu, Y. et al. Time-resolved EPR revealed the formation, structure, and reactivity of N-centered radicals in an electrochemical C(sp3)–H arylation reaction. J. Am. Chem. Soc. 143, 20863–20872 (2021).
Dong, X.-C., Roeckl, J. L., Waldvogel, S. R. & Morandi, B. Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science 371, 507–514 (2021).
Feng, T., Wang, S., Liu, Y., Liu, S. & Qiu, Y. Electrochemical desaturative β-acylation of cyclic N-aryl amines. Angew. Chem. Int. Ed. 61, e202115178 (2022).
Zhong, T., Chen, Z., Yi, J., Lu, G. & Weng, J. Recent progress in the synthesis of sulfonyl fluorides for SuFEx click chemistry. Chin. Chem. Lett. 32, 2736–2750 (2021).
Lou, T. S.-B. & Willis, M. C. Sulfonyl fluorides as targets and substrates in the development of new synthetic methods. Nat. Rev. Chem. 6, 146–162 (2022).
Bianchi, T. A. & Cate, L. Phase transfer catalysis. Preparation of aliphatic and aromatic sulfonyl fluorides. J. Org. Chem. 42, 2031–2032 (1977).
Laudadio, G. et al. Sulfonyl fluoride synthesis through electrochemical oxidative coupling of thiols and potassium fluoride. J. Am. Chem. Soc. 141, 11832–11836 (2019).
Davies, A. T., Curto, J. M., Bagley, S. W. & Willis, M. C. One-pot palladium-catalyzed synthesis of sulfonyl fluorides from aryl bromides. Chem. Sci. 8, 1233–1237 (2017).
Zhang, L., Cheng, X. & Zhou, Q.-L. Electrochemical synthesis of sulfonyl fluorides with triethylamine hydrofluoride. Chin. J. Chem. 40, 1687–1692 (2022).
Lo, P. K. T., Chen, Y. & Willis, M. C. Nickel(II)-catalyzed synthesis of sulfinates from aryl and heteroaryl boronic acids and the sulfur dioxide surrogate DABSO. ACS Catal. 9, 10668–10673 (2021).
Magre, M. & Cornella, J. Redox-neutral organometallic elementary steps at bismuth: catalytic synthesis of aryl sulfonyl fluorides. J. Am. Chem. Soc. 143, 21497–21502 (2021).
Lin, Q. et al. Arenesulfonyl fluoride synthesis via copper-free Sandmeyer-type fluorosulfonylation of arenediazonium salts. Chin. J. Chem. 38, 1107–1110 (2020).
Zhong, T. et al. Copper-free Sandmeyer-type reaction for the synthesis of sulfonyl fluorides. Org. Lett. 22, 3072–3078 (2020).
Lou, T. S.-B. et al. Scalable, chemoselective nickel electrocatalytic sulfinylation of aryl halides with SO2. Angew. Chem. Int. Ed. 61, e202208080 (2022).
Kwon, J. & Kim, B. M. Synthesis of arenesulfonyl fluorides via sulfuryl fluoride incorporation from arynes. Org. Lett. 21, 428–433 (2019).
Lou, T. S.-B., Bagley, S. W. & Willis, M. C. Cyclic alkenylsulfonyl fluorides: palladium-catalyzed synthesis and functionalization of compact multifunctional reagents. Angew. Chem. Int. Ed. 58, 18859–18863 (2019).
Verhoest, P. R. et al. Design and fiscovery of a selective small molecule κ opioid antagonist (2-methyl-N-((2’-(pyrrolidin-1-ylsulfonyl)biphenyl-4-yl)methyl)propan-1-amine, PF-4455242). J. Med. Chem. 54, 5868–5877 (2011).
Louvel, D. et al. Metal-free visible-light synthesis of arylsulfonyl fluorides: Scope and mechanism. Chem. Eur. J. 27, 8704–8708 (2021).
Acknowledgements
X.Q.K. acknowledges the financial support of the National Natural Science Foundation of China (22372015, 22202021), and the Changzhou Science and Technology Plan Applied Basic Research Project (CJ20210159). Y.C. acknowledges the financial support of the Jiangsu Higher Education Institutions of China (21KJB530013). X.C. acknowledges the financial support of the National Natural Science Foundation of China (22102012, 22272011), Changzhou Science and Technology Plan Applied Basic Research Project (CJ20210129, CZ20220022), and the Jiangsu Higher Education Institutions of China (22KJA150001, 21KJD530003). Z.Y.C. acknowledges the financial support of the National Natural Science Foundation of China (22201062) and Natural Science Foundation of Henan Province (222300420111). S.-F. Ni acknowledges funding from the STU Scientific Research Foundation for Talents (NTF20022).
Author information
Authors and Affiliations
Contributions
Z.C. and X.K. conceived the idea and supervised the whole project. Y.C., X.C., M.C., W.W., and Y-Q.X., carried out the experiments. S.N. and C.M. conducted the DFT calculations. All authors discussed the results, contributed to writing the manuscript, commented on the manuscript, and approved the final version of the manuscript for submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Xu Cheng and Zejiang Li for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kong, X., Chen, Y., Chen, X. et al. Organomediated electrochemical fluorosulfonylation of aryl triflates via selective C–O bond cleavage. Nat Commun 14, 6933 (2023). https://doi.org/10.1038/s41467-023-42699-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42699-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.