Abstract
Quaternary amino acids are one of the essential building blocks and precursors of medicinally important compounds. Various synthetic strategies towards their synthesis have been reported. On the other hand, developing core-structure-oriented cross-dehydrogenative coupling (CDC) reactions, is a largely unsolved problem. Herein, we describe a copper-catalyzed regiodivergent electrochemical CDC reaction of Schiff bases and commercially available hydroquinones to obtain three classes of chiral quaternary amino acid derivatives for the efficient assembly of complex scaffolds with excellent stereocontrol. The electrochemical anodic oxidation process with slow releasing of quinones serves as an internal syringe pump and provides high levels of reaction efficiency and enantiomeric control. The utility of this strategy is highlighted through the synthetic utility in the asymmetric total synthesis of (-)-fumimycin.
Similar content being viewed by others
Introduction
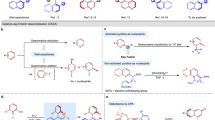
Stereochemically rigid α,α-disubstituted amino acids are a prevalent structural feature in a wide range of biologically active compounds, fueling the ongoing development of a powerful and efficient tool for dramatically increasing the complexity of organic molecules. Fumimycin, which was originally isolated from Aspergillus fumisynnematus, has been shown to exhibit antibacterial and against peptide deformylase inhibitory activity (Fig. 1a)1. Elegant contributions from the groups of Bräse2,3,4, Zhou5, Roche6, Piersanti7, and Watanabe8 have been dedicated to the development of powerful synthetic strategies for the efficient synthesis of fumimycin. Despite significant progress, the assembly of quaternary carbon stereocenters for asymmetric catalytic synthesis of fumimycin in a highly stereoselective manner is still challenging, most likely due to the congested nature of such scaffolds and a lack of reliable synthetic routes for the appropriate installation of the hydroquinone moiety. In this context, the development of cross-dehydrogenative coupling (CDC) methods9, 10 in a catalytic asymmetric fashion would significantly simplify synthetic access to α-quaternary amino acids, which would be invaluable in delivering compound libraries to benefit chemical space in drug design and screening11.
Quinone and its derivatives have long served as useful synthetic precursors which allow the facile synthesis of functionalized aromatic rings12. Accordingly, a plethora of catalytic enantioselective transformations based on the 1,4-addition to the quinone sp2 hybridized carbons to access valuable α-arylated products have been developed13,14,15,16,17. Very recently, seminal works by the List18 and Jørgensen19,20,21 groups revealed asymmetric α-aryloxylation of cyclic ketones or aldehydes via the 1,6-addition of benzoquinones. Nowadays, electrochemical synthesis has received extensive attention as a sustainable way to replace chemical oxidants and reductants22,23,24,25,26,27,28,29,30,31,32,33. The tunability of the potential and electric current, in particular, provides a unique handle for organic electrochemistry to precisely tame redox transformations and exquisitely control the release of reactive intermediates. As a result, many transformations have been developed based on the electro-generated reactive intermediates34. In 2010, Jørgensen and coworkers pioneered the development of enantioselective anodic oxidation/organocatalytic reactions for the α-arylation of aldehydes with in situ generated quinones35. On the other hand, the combination of chiral Lewis acid catalysis with the unique reactivity of electrochemically generated intermediates36,37,38,39 has recently shown the potential to offer a valuable platform for the development of a diverse range of stereocontrolled reactions40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57. Thus, we envisioned that the integration of two units of intermediates, including electrochemically generated quinone species and Lewis acid-bound nucleophiles, would offer opportunities in switchable hydroquinone transformations to access site-diverse regioisomers.
The retrosynthetic analysis of (-)-fumimycin, which focuses on directly assembling quaternary carbon stereocenters, allows the design of the synthetic strategy that utilizes the enantioselective coupling of Schiff bases with electrochemically generated quinones, and would offer mechanistically diverse transformations to access valuable chiral α-tertiary amino ester derivatives rationally and predictably (Fig. 1b). Furthermore, well-established N-metallated azomethine ylides, serving as a binding cavity, reveal distinct reactivity patterns and enable the diversity-oriented synthesis of unnatural α-amino acids with consistently high enantioselectivity58, 59. In this work, we describe the chiral copper-catalyzed, highly enantioselective, regiodivergent, electrochemical CDC reactions of Schiff bases with hydroquinone derivatives, providing diverse synthetic routes toward chiral quaternary amino esters with multiple substituent patterns via anodic oxidation and Lewis acid catalysis (Fig. 1c). Notably, the efficiency of this approach is demonstrated by its application to the enantioselective total synthesis of (-)-fumimycin.
Results and discussion
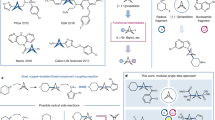
We initiated the study by investigating the electrochemical reaction between racemic ketimine ester 1a and 2,3-dimethylhydroquinone 2a in tetrahydrofuran (THF) (Table 1). The use of chiral box ligand (S,S)-L1 afforded the 1,4-addition product 3a with poor stereocontrol (68% yield, 38% e.e., entry 1). We next evaluated a variety of chiral ligands for this asymmetric electrochemical CDC reaction (entries 2-5), and the product 3a was obtained in 71% yield and 92% e.e. with the use of Phosferrox ligand (S,Sp)-L5 (entry 5). Interestingly, when the reaction was carried out at −10 °C, a switchable reaction pathway that results in kinetic resolution (KR, entry 6) over dynamic kinetic asymmetric transformation (DyKAT, entry 5) of racemic ketimine ester 1a successfully afforded 3a in 57% yield and 92% e.e., as well as the recovery of (S)-1a in 30% yield with 98% e.e. (entry 6). Further studies focused on the evaluation of different ester moieties of ketimine esters 1 (entries 7–9), and the hydroquinone adduct 4a, which can be applied in the analog synthesis of (-)-fumimycin, could be obtained via an initial 1,4-addition and a subsequent intramolecular cyclization. To our surprise, the use of 1-naphthyl ester 1d instead of methyl ester 1a resulted in a successful switchable production of the desired hydroquinone product 4a over 3a, albeit with 81% e.e. (entry 9). Under the electrolytic conditions, the stereoselectivity was further improved with the use of Phosferrox ligand (S,Sp)-L4, leading to the desired product 4a in 80% yield with 95% e.e. (entry 10). Further studies showed that the hydroquinone backbone was also susceptible to the reaction. We successfully switched the α-arylated process to the aryloxylation reaction using bulky 2,6-dimethylhydroquinone 2b under otherwise identical reaction conditions, producing 5a in the majority (entry 11, 78% yield, 92% e.e.).
Mechanistic investigations
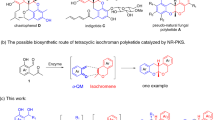
Some preliminary studies were carried out to better understand the mechanism of copper-catalyzed electrochemical regiodivergent reactions (Fig. 2). Initially, control experiments toward KR and DyKAT of racemic ketimine ester 1 were performed (Fig. 2a). Under conditions shown in Table 1, entry 11, (rac)-1a was successfully resolved to its S-enantiomer with considerable stereocontrol (85% e.e., Fig. 2a, entry 1). In contrast, DyKAT of 1-naphthyl ester 1d with 2b went smoothly, generating the desired product 5a in 78% yield and 92% e.e. (entry 2). The reaction involving 1a and 2b in a ratio of 2:1 resulted in 5a′ in 54% yield and 89% e.e., with (S)-1a recovered in 60% e.e. (entry 3); whereas the coupling of 1d with 2b in a ratio of 2:1 led to 5a in 90% yield and 92% e.e. with racemic 1d recovered (entry 4). Subsequently, racemization profiles of (S)-1a (94% e.e.) and (S)-1d (48% e.e.) were investigated in the presence of copper catalytic system and substrate 2b (Fig. 2b). Rapid racemization of Schiff base (S)-1d was observed at −10 °C within 20 min, while (S)-1a remained unaffected over 6 h. More importantly, (S)-1a (94% e.e.) became racemic within 2 h at 10 °C. From these results, we concluded that both the ester moiety of Schiff base 1 and reaction temperature were responsible for the switchable KR and DyKAT process.
As shown in Fig. 2c, the 1H NMR spectrum for the reaction progress of 1d and 2a was monitored under standard conditions (Table 1, entry 10). The full conversion of the starting materials was achieved within 5 h, delivering dehydrogenation adduct 4a smoothly with its e.e. value remaining stable at 95% during the reaction progress. The quinone intermediate 2a′ was not detected until the reaction was nearly complete, which suggested a slow release of quinone intermediate from dimethylhydroquinone 2a. We further investigated the potential pathway arising from the electrochemical-free process with quinone 2a′ (Fig. 2d). Surprisingly, exposing quinone 2a′ with 1d in one portion resulted in the formation of 4a in essentially the same yield, albeit with significantly lower e.e. (Fig. 2d, t = 0 h, 48% e.e.). We speculated that the concentration of quinone would play a key role in this reaction. Thus, the enantiomeric excess of product 4a was measured as a function of the addition time of quinone 2a′. Using a syringe pump, 2a′ was lowly added over 4.5 h, and the results of product 4a (95% e.e.) perfectly matched with the standard electricity-driven protocol (Table 1, entry 10). On this basis, we hypothesized that high levels of reaction efficiency and enantiocontrol can be achieved through an internal syringe pump protocol wherein the controlled release of reactive quinones from the in situ anodic oxidation process of hydroquinones is accomplished.
Taking into account the combined results of mechanistic studies, a proposed mechanism for the Cu-catalyzed regiodivergent CDC reactions is outlined in Fig. 2e. The catalytic cycles begin with the coordination of the copper catalyst to Schiff base 1 to generate the nucleophilic metallated azomethine ylide I. Meanwhile, hydroquinone 2 is electrochemically oxidized at the anode to produce quinone species 2′. Remarkably, mechanistic studies show that an anodic oxidation process exhibiting as the internal syringe pump to the controllable release of quinone species is essential to the stereoselective transformation. The structure of the hydroquinones employed in the reaction defined the initial selectivity between the competing 1,4-addition and 1,6-addition pathways with Cu-coordinated azomethine ylide I. Condensation of I with less steric hydroquinone (such as 2,3-dimethylhydroquinone, 2a) promotes the 1,4-addition to produce the α-arylated products (II or III), and the following reaction process is influenced by different ester groups of Schiff base 1. In other words, methyl ester Schiff base (R3 = Me) would undergo 1,4-addition and electrochemical oxidation to afford quinone product 3 (Path A), whereas naphthyl ester Schiff base (R3 = 1-Nap) is preferred for subsequent intramolecular lactonization to afford product 4 (Path B). Using sterically hindered 2,6-disubstituted hydroquinone 2 as a substrate, 1,6-addition reactivity prevails to provide the final α-aryloxylation product 5 and regenerate the copper catalyst (Path C).
We investigated the generality of this reaction with different substituted Schiff bases after optimizing the reaction conditions for the synthesis of quinone adduct 3 (Fig. 3). As shown in Fig. 3a, a broad range of racemic ketimine esters 1 gave products with high yields and enantioselectivities. Substrates bearing electron-withdrawing or -donating groups on the aromatic rings participated in the transformation, resulting in excellent enantioselectivities (3a-3i). Furthermore, 2-naphthyl and heteroaromatic ketimine esters smoothly underwent this catalytic transformation with high enantioselectivities (3j and 3k). The absolute configuration of adduct 3j was unambiguously assigned by X-ray analysis. Furthermore, methyl 2-thiazoline-4-carboxylate and aldimine ester were effectively converted into the corresponding products (3l and 3m). The generality of the electrochemical CDC reaction in terms of hydroquinone substituents was also explored, and the corresponding products were obtained with satisfactory results (3n and 3o). By lowering the reaction temperature, we were able to expand the purview of this catalytic KR reaction for various racemic Schiff bases 1 (Fig. 3b). The KR of Schiff base 1 with different benzene ring substituents proceeded smoothly, with nearly 50% conversion and with excellent KR results in all instances. Methyl 2-phenyl-2- oxazoline-4-carboxylate 1ap also worked well to deliver quinone product 3p with high levels of stereoselectivity, while unreacted enantiomeric Schiff base (S)-1ap was recovered with 83% e.e.
a Substrate scope for the DyKAT of 1. The reactions were carried out using racemic ketimine ester 1 (0.15 mmol), hydroquinone 2 (0.225 mmol), Cu(MeCN)4BF4 (10 mol%), (S,Sp)-L5 (12 mol%), KOAc (0.3 mmol), nBu4NClO4 (0.07 M), and THF (4 mL) under constant-current conditions in an undivided cell. b Substrate scope for the kinetic resolution of 1. aAt 0 °C. bAt −10 °C. cAt 5 °C. dAt −25 °C. eAt −30 °C. fAt 10 °C.
Next, we explored the scope of the tandem annulation reactions of 1-naphthyl ester 1, for which lactone products 4 are specifically available (Fig. 4). A wide variety of Schiff bases and hydroquinones were investigated under optimized reaction conditions (Table 1, entry 10). Aromatic and heteroaromatic Schiff bases were transformed catalytically with good enantioselectivities (4a-4j). Notably, the opposite enantiomer of 4a can be obtained by using ligand (R,Rp)-L4 under otherwise identical conditions, and the absolute configuration of ent-4a was assigned by single-crystal X-ray diffraction analysis. Further exploration showed that naphthalen-1-yl 2-phenyl-4,5-dihydrothiazole-4-carboxylate was also an appropriate substrate (4k). We then attempted to assess the applicability of this methodology to other types of hydroquinones. The reaction with different substituents on the hydroquinone ring went smoothly, obtaining moderate yields and good enantioselectivities (4l-4o).
Unless otherwise specified, all the reactions were carried out using racemic ketimine ester 1 (0.15 mmol), hydroquinone 2 (0.225 mmol), Cu(MeCN)4BF4 (10 mol%), (S,Sp)-L4 (12 mol%), KOAc (0.3 mmol), nBu4NClO4 (0.07 M), and THF (4 mL) at −10 °C under constant-current conditions in an undivided cell. a(R,Rp)-L4 was used.
In comparison to the prevalence of reactions involving α-arylated reactivity, asymmetric α-aryloxylation for C–O bond formation has received far less attention. A wide range of Schiff bases 1 was investigated with 2,6-disubstituted hydroquinones (Fig. 5a). Substitutions on the benzene ring of Schiff bases had little effect on the reaction (5a-5f). The absolute configuration of 5b was assigned by single-crystal X-ray diffraction analysis. Furthermore, variations in the different substituted groups of hydroquinones 2 had no discernible effect on the reaction outcomes, giving the corresponding α-aryloxylation products in good yields and enantioselectivities (5g-5i). To test the synthetic utility of the electrochemical CDC reactions, optically active α-aryloxylation product 5a can be readily elaborated (Fig. 5b). Under basic conditions, the treatment of compound 5a with different thiols 6 furnished a variety of valuable quaternary thioethers 7 in high yields with maintaining e.e. (7a-7d).
a Isolated O-bound quinol-derivatives. Unless otherwise specified, all the reactions were carried out using racemic ketimine ester 1 (0.15 mmol), hydroquinone 2 (0.225 mmol), Cu(MeCN)4BF4 (10 mol%), (S,Sp)-L4 (12 mol%), KOAc (0.3 mmol), nBu4NClO4 (0.07 M), and THF (4 mL) at −10 °C under constant-current conditions in an undivided cell. b Synthesis of optically active thioesters.
The utility of the current method is further demonstrated by the enantioselective total synthesis of (-)-fumimycin with high stereochemical purity (Fig. 6). Accordingly, 4o was treated with allyl bromide and potassium carbonate to produce allyl ether 8 in 80% yield and 93% e.e. Acidic hydrolysis of the imine group, followed by the treatment with tert-butyl protected acid chloride, generates the corresponding amide 9 without affecting the enantioselectivity. The thermal Claisen rearrangement was performed to afford 10 in DMF at 180 °C2, 3. Subsequent isomerization of the terminal double bond was accomplished under rhodium catalysis, and the desired product 11 was afforded without loss of enantioselectivity. Finally, the methyl group and the tert-butyl ester were cleaved to yield (-)-fumimycin successfully. All the spectroscopic data for our synthetic product are in agreement with those reported in the literature4, 7. Notably, the absolute configuration of (-)-fumimycin is consistent with the X-ray diffraction analysis of 9.
We have developed a Cu-catalyzed asymmetric electrochemical regiodivergent CDC reaction of Schiff bases and hydroquinones. This methodology enables efficient and economic access to a regiodivergent and enantioselective synthesis of valuable amino esters bearing quaternary stereocenters with high functional group tolerance. The regioselectivity could be switched by using the appropriate substrates, and the electrochemical process acts as an internal syringe pump to boost the stereoselectivity, offering an effective platform for future efforts to create complex and value-added quaternary amino acids. The synthetic utility of this asymmetric electrochemical methodology is demonstrated by its application to the total synthesis of (-)-fumimycin.
Methods
General procedure for the synthesis of chiral products 3
In a Schlenk tube equipped with two carbon electrodes, Cu(MeCN)4BF4 (0.015 mmol, 10 mol%) and (S, Sp)-L5 (0.018 mmol, 12 mol%) were stirred in anhydrous THF (2 mL) under argon at 25 °C for 10 min. 1 (0.15 mmol), 2 (0.225 mmol), nBu4NClO4 (0.28 mmol), KOAc (0.3 mmol), and THF (2 mL) were added successively under argon. The constant current (I = 0.4 mA) electrolysis was carried out at 10 °C until complete consumption of the substrate (monitored by TLC, 8–12 h). The solvent was removed under reduced pressure. The residue was purified by silica gel chromatography to afford the desired product 3.
General procedure for the synthesis of chiral products 4
In a Schlenk tube equipped with two carbon electrodes, Cu(MeCN)4BF4 (0.015 mmol, 10 mol%) and (S,Sp)-L4 (0.018 mmol, 12 mol%) were stirred in anhydrous THF (2 mL) under argon at 25 °C for 10 min. 1 (0.15 mmol), 2 (0.225 mmol), nBu4NClO4 (0.28 mmol), KOAc (0.3 mmol), and THF (2 mL) were added successively under argon. The constant current (I = 0.4 mA) electrolysis was carried out at −10 °C until complete consumption of the substrate (monitored by TLC, 6–8 h). The solvent was removed under reduced pressure. The residue was purified by silica gel chromatography to afford the desired product 4.
General procedure for the synthesis of chiral products 5
In a Schlenk tube equipped with two carbon electrodes, Cu(MeCN)4BF4 (0.015 mmol, 10 mol%) and (S,Sp)-L4 (0.018 mmol, 12 mol%) were stirred in anhydrous THF (2 mL) under argon at 25 °C for 10 min. 1 (0.15 mmol), 2 (0.225 mmol), nBu4NClO4 (0.28 mmol), KOAc (0.3 mmol), and THF (2 mL) were added successively under argon. The constant current (I = 0.4 mA) electrolysis was carried out at −10 °C until complete consumption of the substrate (monitored by TLC, 8−12 h). The solvent was removed under reduced pressure. The residue was purified by silica gel chromatography to afford the desired product 5.
Data availability
Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2249495 (3j), CCDC 2249496 (ent-4a), CCDC 2249497 (5b), and CCDC 2249498 (9). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study, including experimental procedures and compound characterization, NMR, and HPLC are available within the Article and its Supplementary Information or from the authors. NMR data in a mnova file format and HPLC traces are available at Zenodo at https://zenodo.org/record/8249500, under the Creative Commons Attribution 4.0 International license.
References
Kwon, Y.-J., Sohn, M.-J., Zheng, C.-J. & Kim, W.-G. Fumimycin: a peptide deformylase inhibitor with an unusual skeleton produced by aspergillus fumisynnematus. Org. Lett. 9, 2449–2451 (2007).
Gross, P. J., Hartmann, C. E., Nieger, M. & Bräse, S. Synthesis of methoxyfumimycin with 1,2-addition to ketimines. J. Org. Chem. 75, 229–232 (2010).
Gross, P. J. & Bräse, S. The total synthesis of (±)-fumimycin. Chem. Eur. J. 16, 12660–12667 (2010).
Gross, P. J., Furche, F., Nieger, M. & Bräse, S. Asymmetric total synthesis of (+)-fumimycin via1,2-addition to ketimines. Chem. Commun. 46, 9215–9217 (2010).
Zhou, Z. et al. A concise synthesis of (±)-methoxyfumimycin ethyl ester. J. Chem. Res. 38, 378–380 (2014).
Zaghouani, M., Bögeholz, L. A. K., Mercier, E., Wintermeyer, W. & Roche, S. P. Total synthesis of (±)-fumimycin and analogues for biological evaluation as peptide deformylase inhibitors. Tetrahedron 75, 3216–3230 (2019).
Retini, M., Bartolucci, S., Bartoccini, F., Mari, M. & Piersanti, G. Concise and convergent enantioselective total syntheses of (+)- and (−)-fumimycin. J. Org. Chem. 84, 12221–12227 (2019).
Kishimoto, S. et al. Reactive azlactone intermediate drives fungal secondary metabolite cross-pathway generation. J. Am. Chem. Soc. 145, 3221–3228 (2023).
Li, C.-J. Cross-dehydrogenative coupling (CDC): exploring C−C bond formations beyond functional group transformations. Acc. Chem. Res. 42, 335–344 (2009).
Liu, L. Hydride-abstraction-initiated catalytic stereoselective intermolecular bond-forming processes. Acc. Chem. Res. 55, 3537–3550 (2022).
Potowski, M., Schürmann, M., Preut, H., Antonchick, A. P. & Waldmann, H. Programmable enantioselective one-pot synthesis of molecules with eight stereocentres. Nat. Chem. Biol. 8, 428–430 (2012).
Wendlandt, A. E. & Stahl, S. S. Quinone-catalyzed selective oxidation of organic molecules. Angew. Chem. Int. Ed. 54, 14638–14658 (2015).
Alemán, J., Richter, B. & Jørgensen, K. A. Organocatalytic highly enantioselective α-arylation of β-ketoesters. Angew. Chem. Int. Ed. 46, 5515–5519 (2007).
Alemán, J., Cabrera, S., Maerten, E., Overgaard, J. & Jørgensen, K. A. Asymmetric organocatalytic α-arylation of aldehydes. Angew. Chem. Int. Ed. 46, 5520–5523 (2007).
Zhang, Y.-C., Zhao, J.-J., Jiang, F., Sun, S.-B. & Shi, F. Organocatalytic asymmetric arylative dearomatization of 2,3-disubstituted indoles enabled by tandem reactions. Angew. Chem. Int. Ed. 53, 13912–13915 (2014).
Chen, Y.-H., Qi, L.-W., Fang, F. & Tan, B. Organocatalytic atroposelective arylation of 2-naphthylamines as a practical approach to axially chiral biaryl amino alcohols. Angew. Chem. Int. Ed. 56, 16308–16312 (2017).
Chen, Y.-H. et al. Atroposelective synthesis of axially chiral biaryldiols via organocatalytic arylation of 2‑naphthols. J. Am. Chem. Soc. 137, 15062–15065 (2020).
Shevchenko, G. A., Oppelaar, B. & List, B. An unexpected α-oxidation of cyclic ketones with 1,4-benzoquinone by enol catalysis. Angew. Chem. Int. Ed. 57, 10756–10759 (2018).
Blom, J. et al. Umpolung strategy for α-functionalization of aldehydes for the addition of thiols and other nucleophiles. Angew. Chem. Int. Ed. 58, 17856–17862 (2019).
Lamhauge, J. N., Corti, V., Liu, Y. & Jørgensen, K. A. Enantioselective α-etherification of branched aldehydes via an oxidative umpolung strategy. Angew. Chem. Int. Ed. 60, 18728–18733 (2021).
Rezayee, N. M., Lamhauge, J. N. & Jørgensen, K. A. Organocatalyzed cross-nucleophile couplings: umpolung of catalytic enamines. Acc. Chem. Res. 55, 1703–1717 (2022).
Yoshida, J., Kataoka, K., Horcajada, R. & Nagaki, A. Modern strategies in electroorganic synthesis. Chem. Rev. 108, 2265–2299 (2008).
Francke, R. & Little, R. D. Redox catalysis in organic electrosynthesis: basic principles and recent developments. Chem. Soc. Rev. 43, 2492–2521 (2014).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Feng, R., Smith, J. A. & Moeller, K. D. Anodic cyclization reactions and the mechanistic strategies that enable optimization. Acc. Chem. Res. 50, 2346–2352 (2017).
Wiebe, A. et al. Electrifying organic synthesis. Angew. Chem. Int. Ed. 57, 5594–5619 (2018).
Möhle, S. et al. Modern electrochemical aspects for the synthesis of value-added organic products. Angew. Chem. Int. Ed. 57, 6018–6041 (2018).
Jiang, Y., Xu, K. & Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 118, 4485–4540 (2018).
Moeller, K. D. Using physical organic chemistry to shape the course of electrochemical reactions. Chem. Rev. 118, 4817–4833 (2018).
Nutting, J. E., Rafiee, M. & Stahl, S. S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 118, 4834–4885 (2018).
Tang, S., Liu, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution: a green and sustainable way for bond formation. Chem. 4, 27–45 (2018).
Kärkäs, M. D. Electrochemical strategies for C-H functionalization and C-N bond formation. Chem. Soc. Rev. 47, 5786–5865 (2018).
Meyer, T. H., Choi, I., Tian, C. & Ackermann, L. Powering the future: how can electrochemistry make a difference in organic synthesis? Chem. 6, 2484–2496 (2020).
Yang, Z., Shi, W., Alhumade, H., Yi, H. & Lei, A. Electrochemical oxidative C(sp3)–H cross-coupling with hydrogen evolution. Nat. Synth. 2, 217–230 (2023).
Jensen, K. L., Franke, P. T., Nielsen, L. T., Daasbjerg, K. & Jørgensen, K. A. Anodic oxidation and organocatalysis: direct regio- and stereoselective access to meta-substituted anilines by α-arylation of aldehydes. Angew. Chem. Int. Ed. 49, 129–133 (2010).
Ghosh, M., Shinde, V. S. & Rueping, M. A review of asymmetric synthetic organic electrochemistry and electrocatalysis: concepts, applications, recent developments and future directions. Beilstein J. Org. Chem. 15, 2710–2746 (2019).
Lin, Q., Li, L. & Luo, S. Asymmetric electrochemical catalysis. Chem. Eur. J. 25, 10033–10044 (2019).
Chang, X., Zhang, Q. & Guo, C. Asymmetric electrochemical transformations. Angew. Chem. Int. Ed. 59, 12612–12622 (2020).
Wang, X., Xu, X., Wang, Z., Fang, P. & Mei, T. Advances in asymmetric organotransition metal-catalyzed electrochemistry. Chin. J. Org. Chem. 40, 3738–3747 (2020).
Fu, N., Li, L., Yang, Q. & Luo, S. Catalytic asymmetric electrochemical oxidative coupling of tertiary amines with simple ketones. Org. Lett. 19, 2122–2125 (2017).
DeLano, T. J. & Reisman, S. E. Enantioselective electroreductive coupling of alkenyl and benzyl halides via nickel catalysis. ACS Catal. 9, 6751–6754 (2019).
Huang, X., Zhang, Q., Lin, J., Harms, K. & Meggers, E. Electricity-driven asymmetric Lewis acid catalysis. Nat. Catal. 2, 34–40 (2019).
Zhang, Q., Chang, X., Peng, L. & Guo, C. Asymmetric Lewis acid catalyzed electrochemical alkylation. Angew. Chem. Int. Ed. 58, 6999–7003 (2019).
Fu, N. et al. New bisoxazoline ligands enable enantioselective electrocatalytic cyanofunctionalization of vinylarenes. J. Am. Chem. Soc. 141, 14480–14485 (2019).
Qiu, H. et al. Enantioselective Ni-catalyzed electrochemical synthesis of biaryl atropisomers. J. Am. Chem. Soc. 142, 9872–9878 (2020).
Dhawa, U. et al. Enantioselective pallada-electrocatalyzed C−H activation by transient directing groups: expedient access to helicenes. Angew. Chem. Int. Ed. 59, 13451–13457 (2020).
Li, L., Li, Y., Fu, N., Zhang, L. & Luo, S. Catalytic asymmetric electrochemical α-arylation of cyclic β-ketocarbonyls with anodic benzyne intermediates. Angew. Chem. Int. Ed. 59, 14347–14351 (2020).
Gao, P.-S. et al. CuII/TEMPO-catalyzed enantioselective C(sp3)–H alkynylation of tertiary cyclic amines through Shono-type oxidation. Angew. Chem. Int. Ed. 59, 15254–15259 (2020).
Song, L. et al. Dual electrocatalysis enables enantioselective hydrocyanation of conjugated alkenes. Nat. Chem. 12, 747–754 (2020).
Wang, Z.-H. et al. TEMPO-enabled electrochemical enantioselective oxidative coupling of secondary acyclic amines with ketones. J. Am. Chem. Soc. 143, 15599–15605 (2021).
Zhang, Q., Liang, K. & Guo, C. Asymmetric electrochemical arylation in the formal synthesis of (+)-amurensinine. CCS Chem. 3, 338–347 (2021).
Xiong, P., Hemming, M. & Ivlev, S. I. & Meggers, E. Electrochemical enantioselective nucleophilic α-C(sp3)–H alkenylation of 2-acyl imidazoles. J. Am. Chem. Soc. 144, 6964–6971 (2022).
Liang, K., Zhang, Q. & Guo, C. Nickel-catalyzed switchable asymmetric electrochemical functionalization of alkenes. Sci. Adv. 8, eadd7134 (2022).
Cai, C.-Y. et al. Photoelectrochemical asymmetric catalysis enables site- and enantioselective cyanation of benzylic C–H bonds. Nat. Catal. 5, 943–951 (2022).
Zhang, Q., Liang, K. & Guo, C. Enantioselective nickel-catalyzed electrochemical radical allylation. Angew. Chem. Int. Ed. 61, e202210632 (2022).
Tan, X., Wang, Q. & Sun, J. Electricity-driven asymmetric bromocyclization enabled by chiral phosphate anion phase-transfer catalysis. Nat. Commun. 14, 357 (2023).
Münchow, T. V., Dana, S., Xu, Y., Yuan, B. & Ackermann, L. Enantioselective electrochemical cobalt-catalyzed aryl C–H activation reactions. Science 379, 1036–1042 (2023).
Tang, S., Zhang, X., Sun, J., Niu, D. & Chruma, J. J. 2-Azaallyl anions, 2-azaallyl cations, 2-azaallyl radicals, and azomethine ylides. Chem. Rev. 118, 10393–10457 (2018).
Wei, L., Chang, X. & Wang, C.-J. Catalytic asymmetric reactions with N-metallated azomethine ylides. Acc. Chem. Res. 53, 1084–1100 (2020).
Acknowledgements
The authors acknowledge financial support from the National Natural Science Foundation of China (grant no. 21971227, 22222113), CAS Project for Young Scientists in Basic Research (YSBR-054), and the Fundamental Research Funds for the Central Universities (WK9990000090, WK9990000111).
Author information
Authors and Affiliations
Contributions
C.G. conceived the project. T.X. performed the experiments and analyzed the data. J.H., J.L. and L.P. synthesized some of the substrates and ligands. C.G. and J.S. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, T., Huang, J., Li, J. et al. Cu-catalyzed asymmetric regiodivergent electrosynthesis and its application in the enantioselective total synthesis of (-)-fumimycin. Nat Commun 14, 6749 (2023). https://doi.org/10.1038/s41467-023-42603-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42603-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.