Abstract
The phenyl ring is a basic structural element in chemistry. Here, we show the design, synthesis, and validation of its new saturated bioisostere with improved physicochemical properties − 2-oxabicyclo[2.2.2]octane. The design of the structure is based on the analysis of the advantages and disadvantages of the previously used bioisosteres: bicyclo[1.1.1]pentane, bicyclo[2.2.2]octane, and cubane. The key synthesis step is the iodocyclization of cyclohexane-containing alkenyl alcohols with molecular iodine in acetonitrile. 2-Oxabicyclo[2.2.2]octane core is incorporated into the structure of Imatinib and Vorinostat (SAHA) drugs instead of the phenyl ring. In Imatinib, such replacement leads to improvement of physicochemical properties: increased water solubility, enhanced metabolic stability, and reduced lipophilicity. In Vorinostat, such replacement results in a new bioactive analog of the drug. This study enhances the repertoire of available saturated bioisosteres of (hetero)aromatic rings for the use in drug discovery projects.
Similar content being viewed by others
Introduction
The phenyl ring is a basic structural element in chemistry. It is one of the most common structural motifs in natural products1 and bioactive compounds2,3. Moreover, more than five hundred drugs contain a fragment of para-substituted phenyl ring (Fig. 1a, b)4, including the well-known to everyone Paracetamol. However, organic compounds with more than two phenyl rings often suffer from poor solubility5,6,7.
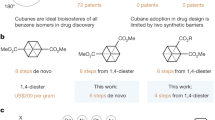
a The para-substituted phenyl ring is a part of >500 drugs and agrochemicals. Bicyclo[1.1.1]pentanes, bicyclo[2.2.2]octanes, and cubane as saturated bioisosteres of the para-substituted phenyl ring. b Bioactive derivatives of bicyclo[1.1.1]pentanes, bicyclo[2.2.2]octanes, and cubane are described in >3000 patents. c Aim of this work: replacement of the para-substituted phenyl ring in bioactive compounds with 2-oxabicyclo[2.2.2]heptane. d Previous syntheses of 2-oxabicyclo[2.2.2]heptane by Singh, Fukuda (2014)50 and Harrison (2019)54.
In 2012, however, Stepan and colleagues showed that a replacement of the central phenyl ring in a γ-secretase inhibitor with the bicyclo[1.1.1]pentane improved its physicochemical properties and retained bioactivity8,9,10,11. Later, analogous replacements were undertaken with cubane12,13,14,15,16,17,18,19, and bicyclo[2.2.2]octane (Fig. 1a, b)20,21,22. Therefore, during the past decade, these scaffolds proved to be useful in drug discovery, medicinal chemistry, and supramolecular chemistry23,24,25,26,27,28,29,30,31. Replacement of the ortho- and meta-substituted phenyl rings in bioactive compounds with saturated bioisosteres was also recently achieved26,27,28,29,30,31. Recent studies, however, showed that all three bioisosteres had drawbacks. In bicyclo[1.1.1]pentane, the most popular among them today32,33,34,35,36,37,38,39,40, the distance between two bridgehead carbon atoms (C-C) is 1.8 Å, which is ca. 35% shorter than that in the para-substituted phenyl ring (2.8 Å). Bicyclo[2.2.2]octane has a closer C-C distance (2.6 Å), but higher lipophilicity41. Cubane, in turn, was recently demonstrated to be unstable under contact with transition metals42,43, under mechanochemical treatment or heating44.
In this work, we have rationally designed, synthesized, and characterized the new bioisostere of the phenyl ring – 2-oxabicyclo[2.2.2]octane (Fig. 1c).
Interestingly, 2-oxabicyclo[2.2.2]octane core has been known in the literature, but not in the context of phenyl bioisostere. Chemists used it as a starting material in organic synthesis;45,46 and in medicinal chemistry47,48,49 as an analog of 4-aminopiperidine50,51,52,53 or cyclohexane54,55. Also, 2-oxabicyclo[2.2.2]octane containing molecules exhibited a broad range of biological activities: estrogen receptor-beta agonists47, myeloperoxidase inhibitors48, antibacterial agents49,50,51,52,53, DGAT1 Inhibitors54, and RORγt agonists55.
Results
Design
In the design of the improved phenyl bioisostere, we first needed to keep the advantages of the previously used cores: their conformational rigidity, metabolic stability, non-chirality, and collinearity of vectors (φ = 180°). At the same time, we needed to address their drawbacks: C-C distance, and lipophilicity. Considering the possible saturated structures (for the details of the design, please, see Supplementary Iinformation, page 5, Supplementary Fig. 1.), we decided to select the bicyclo[2.2.2]octane scaffold, because of its appropriate C-C distance, and decorate it with an oxygen atom. In particular, replacing one carbon atom with oxygen would give 2-oxabicyclo[2.2.2]octane with similar geometry and reduced lipophilicity (Fig. 1c). Also, this structure should be chemically stable as a simple derivative of tetrahydropyran.
Optimization
Synthesis of the 2-oxabicyclo[2.2.2]octane core has been previously reported. In 2014, Singh and Fukuda obtained compound 1 from diethyl malonate (2) in 15 steps using alkylation as a key reaction (Fig. 1d)50. In 2019, Harrison synthesized compound 3 from ester 4 in six steps employing an intramolecular Michael addition (Fig. 1d)54. The latter approach was limited only to aromatic substituents. We, however, needed a general modular method that would give 2-oxabicyclo[2.2.2]octanes with one or two functional groups that could be subsequently modified to obtain a wide variety of derivatives - bioisosteres of the mono- and para-substituted phenyl rings.
Previously, we showed that smaller 2-oxabicyclo[2.1.1]hexane could be assembled via the iodocyclization reaction of the corresponding cyclobutane alkenyl alcohols56. The reaction proceeded with I2/NaHCO3 in the mixture of water and MeOtBu at room temperature. We hoped that similar cyclization would also take place with cyclohexane 5 (please, see its preparation below). However, under analogous conditions the expected product 6 was not formed (Table 1, entry 1). We repeated the reaction several times varying the time and the temperature, however, with the same negative outcome (Table 1, entries 2-4). The addition of the iodine molecule to the double C=C bond did take place, but the cyclization failed to occur.
Subsequently, we realized that in contrast to the already conformationally preorganized small cyclobutane, the larger and more flexible cyclohexane ring should adopt the highly energetic boat conformation first (Table 1). The resulting entropic penalty seems to prevent the cyclization from occuring. We also tried other combinations of solvents with no success, however (Table 1, entries 5-8). Finally, we used solely dipolar aprotic solvents. Indeed, in dimethyl formamide, the formation of traces of the needed product was finally seen (Table 1, entry 9). A similar result was observed in dimethyl sulfoxide and N-methyl pyrrolidone (Table 1, entries 10, 11). In acetonitrile, the transformation proceeded cleaner, and iodide 6 was obtained in a 56% yield (Table 1, entry 12). Performing the reaction under heating (Table 1, entry 13) or employing bromine (Br2; Table 1, entry 14) did not improve the yield.
Scalable synthesis
Having a working procedure in hand, we studied its scalability. The whole synthesis scheme commenced from the commercially available ketoester 7 (ca. 3€/g, Fig. 2). Wittig reaction gave alkene 8 in 59% yield. Treatment of the latter with LDA/methyl formate followed by the reduction of the intermediate aldehyde with NaBH4 gave alcohol 5 in 86% combined yield. Finally, the key iodocyclization was attempted on a multigram scale. Pure iodide 6 was obtained as a white crystalline solid after column chromatography with a 36% yield. Despite a rather moderate yield, this protocol allowed us to obtain 135 g of the product in a single run.
Scope
Next, we studied the generality of the developed protocol. Treatment of alkene 8 with LDA/acetaldehyde gave the intermediate alcohol that was used in the subsequent iodocyclization under the developed conditions. The expected iodide 9 was isolated in 50% yield after column chromatography (Fig. 3a). Initially, we isolated the intermediate alcohol, but subsequently, we understood that performing the two-step procedure with a simple solvent swap ensured a better yield of the final product.
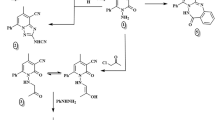
a Synthesis of 2-oxabicyclo[2.2.2]octanes with three exit vectors (for products 10–29, ethyl ester analog of alkene 8 was used). b Synthesis of 2-oxabicyclo[2.2.2]octanes with one and two exit vectors. X-ray crystal structure of compound 30b (carbon – white, oxygen – red, iodine - violet). Hydrogen atoms are omitted for clarity. c Iodocyclization of alkenes 34, 36, and 38. d, Synthesis of 2-azabicyclo[2.2.2]octane 43.
The reaction with aliphatic (10–12), aromatic (13–17), and heterocyclic (18–27) aldehydes gave the corresponding 2-oxabicyclo[2.2.2]octanes in good yields. Functional groups such as nitro, trifluoromethoxy, trifluoromethyl, nitrile, and halogen atoms tolerated the reaction conditions. The protocol was not without limitations, however. We could not obtain products 28, and 29 with thiazole and triazole heterocycles, due to the formation of complex mixtures (Fig. 3a). Ketones could also be used as electrophiles instead of aldehydes. As a representative example, the reaction of alkene 8 with LDA/acetone followed by iodocyclization gave dimethyl-substituted product 30 in 81% yield. The structure of 30 was confirmed by X-ray crystallographic analysis (Fig. 3b, Supplementary Data 1). A reduction of 8 followed by iodocyclization gave iodide 31 in 58% yield. Interestingly, the cyclization was not efficient at room temperature, and required heating. Alkylation of 8 with MeI or BnOCH2Cl followed by reduction and iodocyclization gave the disubstituted products 32, 33 in 59-64% yield (Fig. 3b).
Trisubstituted exocyclic alkenes also afforded the desired 2-oxabicyclo[2.2.2]octane skeleton. For example, the iodocyclization of alkene 34 under the standard conditions gave iodide 35 in 67% yield (Fig. 3c). Alkene 36 provided iodide 37 in 50% yield. Endocyclic alkene 38, however, gave the isomeric core - 6-oxabicyclo[3.2.1]octane 3957.
We also tried to assemble a 2-azabicyclo[2.2.2]octane skeleton using the developed strategy. An attempted iodocyclization of alkene 40 did not lead to the formation of the cyclic iodide 41 neither at room temperature nor under heating (Fig. 3d). However, the analogous reaction of the bridgehead-substituted alkene 42 at room temperature did give the needed product 43 in 31% yield. Under heating, the yield was improved to 41%.
Modifications
Several representative modifications of the obtained iodides were undertaken to obtain various mono- and bifunctional 2-oxabicyclo[2.2.2]octanes ready for direct use in medicinal chemistry projects. Treatment of iodide 31 with potassium thioacetate followed by oxidation with NCS gave aliphatic sulfonyl chloride 44 in 85% yield. The reaction of 31 with potassium acetate and the subsequent alkali hydrolysis provided valuable alcohol 45. Oxidation of the latter afforded carboxylic acid 46 in 89% yield (Fig. 4).
Hydrogenative reduction of the C-I bond in iodide 6 followed by saponification of the ester group gave methyl acid 47. The Curtius reaction of the latter resulted in amine 48. The reaction of iodide 6 with LiAlH4 gave alcohol 49 in 90% yield. O-Mesylation and the subsequent reaction with LiBr provided bromide 50. Swern oxidation of alcohol 49 gave aldehyde 51 in 63% yield. Isomeric methyl-substituted 2-oxabicyclo[2.2.2]octanes were obtained from iodide 32. Its reaction with sodium azide followed by the reduction formed amine 52. The reaction of iodide 32 with potassium acetate and hydrolysis gave alcohol 53 - isomer of alcohol 49. Oxidation of 53 formed carboxylic acid 54 - isomer of acid 47. Sulfonyl chloride 55 was also obtained from iodide 32 via a two-step procedure (Fig. 4).
From iodide 6 we also synthesized various bifunctional linkers for incorporation into bioactive compounds instead of the para-substituted phenyl ring. Saponification of ester 6 provided carboxylic acid 56 in 90% yield. The subsequent Curtius reaction afforded N-Boc iodide 57 in 87% yield. The structure of 57 was confirmed by X-ray crystallographic analysis (Supplementary Data 2). The reaction of the latter with potassium acetate, followed by ester hydrolysis (via 58) and N-Boc acidic deprotection gave amino alcohol 59. Oxidation of the alcohol group in 58 gave N-Boc protected amino acid 60 – a saturated analog of the para-aminobenzoic acid. The reaction of iodide 57 with NaN3 (via azide 61) followed by reduction of the azide group formed diamine 62. The reaction of iodide 6 with NaN3 (via azide 63), the subsequent reduction (via 64), N-Boc protection, and saponification gave another N-Boc protected amino acid 65. The Curtius reaction of the latter provided N-Boc diamine 66 – isomer of diamine 62. The reaction of iodide 6 with sodium azide followed by extensive reduction of the intermediate azide with LiAlH4 gave amino alcohol 67. The structure of 67 was confirmed by X-ray crystallographic analysis (Supplementary Data 3). The reaction of iodide 6 with potassium acetate (via 68) followed by saponification of the ester group and oxidation gave linker 69. Its structure was also confirmed by X-ray crystallographic analysis (Supplementary Data 4). Worth noting that all the above-described syntheses depicted in Fig. 4 were realized on a multigram scale.
Alkylation of 4-bromothiophenol with iodide 32 followed by oxidation of the intermediate sulfide gave sulfone 70 in 54% yield over two steps (Fig. 4). Sulfonamide 71 was obtained in 44% yield from amine 48. Cu-catalyzed click reaction between azide 63 and 3-ethynylquinoline smoothly provided triazole 72. Condensation of acid 73 with N-hydroxyphthalimide (NHPI) in the presence of N,N’-diisopropylcarbodiimide (DIC) gave the activated ester 74 (Fig. 4). Ni-mediated Barton decarboxylation58 of the latter with PhSiH3 was performed next to provide ester 75.
Ni-Mediated C-C cross-coupling of iodide 31 with PhMgBr gave 2-oxabicyclo[2.2.2]octane 76 in 67% yield. The reaction of aldehyde 51 with p-toluenesulfonyl hydrazide gave the intermediate hydrazone that upon treatment with the cyclopropylboronic acid and pinacol provided organoboron derivative 7759. Condensation of acid 47 with N-hydroxyphthalimide gave the activated ester 78. Its structure was confirmed by X-ray crystallographic analysis (Supplementary Data 5). Cu-Catalyzed decarboxylative borylation60 of ester 78 gave organoboron derivative 79 (Fig. 4).
Chemical stability
We also examined the thermal and chemical stability of the synthesized 2-oxabicyclo[2.2.2]octanes. As representative examples, we selected three molecules: isomeric acids 47, 54, and amine 52. All 2-oxabicyclo[2.2.2]octanes were crystalline solids that were stable in air. We stored them in stock at room temperature in closed vials and observed no changes according to 1H NMR after one year. Also, the compounds remained stable even under heating at 100 °C for five minutes. Treatment of the selected 2-oxabicyclo[2.2.2]octanes with aq. 1 M HCl, or aq. 1 M NaOH at room temperature for 1 h resulted in no decomposition either.
Crystallographic analysis
Next, we compared the geometric properties of 2-oxabicyclo[2.2.2]octanes with those of the para-substituted phenyl ring, and the previously used bioisosteres - bicyclo[2.2.2]octanes. For this purpose, we measured two C-C distances r and d to see the overall similarity of cores; and two angles φ1 and φ2 to estimate the collinearity of exit vectors (Fig. 5a).
a Definition of distances r, d and angles φ1, φ2 (2-oxabicyclo[2.2.2]octane core is shown as example). b Geometric parameters r, d and φ1, φ2 for para-substituted phenyl ring (Imatinib drug), its known saturated bioisosteres 80–82 and the new saturated bioisosteres 30, 69. aData is taken from ref. 64. bData is taken from ref. 61. cData is taken from ref. 62. dData is taken from ref. 63. Two individual molecules of Imatinib (A and B) are present in the crystal lattice.
We calculated the values of r, d, φ1, and φ2 of 2-oxabicyclo[2.2.2]octanes from the X-ray data of compounds 30, 69. The related parameters for bicyclo[2.2.2]octanes 8061, 8162, and 8263 were calculated from their X-ray data published in the literature (Fig. 5b). The corresponding parameters for the para-substituted phenyl ring were calculated from the reported crystal structure of the anticancer drug Imatinib64. Analysis of this data revealed that the geometric properties of 2-oxabicyclo[2.2.2]octanes were indeed very similar to those of the para-substituted phenyl ring. The distance r in 2-oxabicyclo[2.2.2]octanes was ca. 0.3 Å shorter than that in the para-phenyl ring: 2.54–2.56 Å vs 2.88–2.89 Å (para-phenyl). The distance d between substituents in 2-oxabicyclo[2.2.2]octanes was also ca. 0.3 Å shorter than that in the para-phenyl ring: 5.56–5.58 Å vs 5.90–5.93 Å (para-phenyl). The difference in collinearity of vectors was insignificant, as angles φ1 and φ2 were almost identical in both scaffolds: 176-177° vs 178-179° (para-phenyl). Interestingly, even in the para-substituted phenyl ring in Imatinib in the crystal phase, the observed angles φ1 and φ2 deviated from the ideal value of 180°: 176-179°. It must be noted, that all parameters, - r, d, φ1 and φ2, - were also almost identical in both bicyclo[2.2.2]octanes (80–82) and 2-oxabicyclo[2.2.2]octanes (30, 69) (Fig. 5b).
In short summary, the replacement of the methylene group for an oxygen atom in the bicyclo[2.2.2]octane core did not affect its three-dimensional geometry. Moreover, the formed 2-oxabicyclo[2.2.2]octane core resembled well the para-substituted phenyl ring, as the geometric parameters r, d, φ1, and φ2 remained very similar (please, see SI, page 277, Supplementary Fig. 8).
The acidity of functional groups
We also studied the influence of the replacement of the methylene group for an oxygen atom in the bicyclo[2.2.2]octane skeleton on the electronic properties. Towards this goal, we measured experimental pKa values of isomeric 2-oxabicyclo[2.2.2]octane carboxylic acids 47 and 54, bicyclo[2.2.2]octane carboxylic acid 84, and para-methyl benzoic acid (83) as a reference (Fig. 6). Replacement of the methylene group in 84 for the oxygen atom at the distal γ-position notably increased its acidity from pKa = 5.6 to 4.4 (47). However, analogous replacement at the β-position increased the acidity even more to pKa = 4.1 (54).
Important to mention that the acidity of aromatic carboxylic acid 83 and 2-oxabicyclo[2.2.2]octane 47 were almost identical (Fig. 6). The replacement of the phenyl ring in acid 83 with the bicyclo[2.2.2]octane core reduced the acidity: pKa = 4.5 (83) vs 5.6 (84). However, incorporation of the β-oxygen atom into the latter ideally restored it: pKa = 4.4 (47). Because the acidity/basicity of functional groups is often responsible for the potency, selectivity, and toxicity of bioactive compounds65, the fine-tuning of the pKa by replacing the phenyl ring with isomeric 2-oxabicyclo[2.2.2]octanes could become a preferred solution.
Incorporation into drugs
To demonstrate the practical utility of the 2-oxabicyclo[2.2.2]octane scaffold, we incorporated it into the structure of anticancer drugs Imatinib, and Vorinostat (SAHA) instead of the para- and mono-substituted phenyl rings, correspondingly (Figs. 7 and 8).
Solubility: experimental kinetic solubility in phosphate-buffered saline, pH 7.4 (µM). clogP: calculated lipophilicity. logD (7.4): experimental distribution coefficient in n-octanol/phosphate-buffered saline, pH 7.4. Reliable logD measured were obtained within a range of 1.0–4.5. CLint clearance intrinsic: experimental metabolic stability in human liver microsomes (µl/min/mg). t1/2 (min) experimental half-time of a metabolic decomposition.
The reaction of iodide 56 with N-methyl piperazine, followed by acylation with the substituted aniline gave compound 85 – a saturated analog of Imatinib (Fig. 7). For comparison, we also synthesized compound 86 with the bicyclo[2.2.2]octane core (please, see SI, pages 52-54). The commercialized drug Imatinib is used in practice as a mesylate salt. However, to estimate the impact of the replacement of the phenyl ring with bioisosteres on the physicochemical properties, we prepared and studied all three compounds, - 85, 86, Imatinib, - as free bases.
From amine 87, in three steps we synthesized compound 88 - a saturated analog of Vorinostat (Fig. 8). For comparison, we also obtained analog 89 with the bicyclo[2.2.2]octane skeleton (please, see SI, pages 56, 57).
Physicochemical properties
Replacement of the para-substituted phenyl ring in Imatinib by bicyclo[2.2.2]octane (86) decreased the water solubility by more than three times (Fig. 7). However, the incorporation of the 2-oxabicyclo[2.2.2]octane (85) in Imatinib increased the solubility close to the original values: 351 µM (Imatinib) vs 113 µM (86) vs 389 µM (85).
To study the replacement of the phenyl ring with saturated bioisosteres on lipophilicity, we used two characteristics: calculated (clogP)66 and experimental (logD) lipophilicities. Incorporation of bicyclo[2.2.2]octane in sted of the phenyl ring resulted in a decrease of clogP: 4.5 (Imatinib) vs 3.6 (86). The incorporation of 2-oxabicyclo[2.2.2]octane led to an even further decrease of clogP: 2.6 (85). A somewhat similar trend was observed with the experimental lipophilicity, logD. While the incorporation of the bicyclo[2.2.2]octane core into Imatinib did not significantly affect it; incorporation of the 2-oxabicyclo[2.2.2]octane core reduced it by ca. one unit, logD: 2.6 (Imatinib) vs 2.7 (86) vs 1.8 (85).
The effect of saturated bioisosteres on metabolic stability was studied next. The incorporation of both bicyclo[2.2.2]octane (86) and 2-oxabicyclo[2.2.2]octane (85) into Imatinib, increased the metabolic stability in human liver microsomes: CLint (mg/(min•μL)) = 28 (Imatinib) vs 16 (86) vs 19 (85) (Fig. 7). Moreover, incorporation of the 2-oxabicyclo[2.2.2]octane core (85) into Imatinib increased the life half time by almost 50%: t1/2 (min) = 60 (Imatinib) vs 87 (85).
In summary, the replacement of the para-substituted phenyl ring in Imatinib with common bicyclo[2.2.2]octane core (86) led to an undesired three-times decrease in water solubility. At the same time, analogous replacement with 2-oxabicyclo[2.2.2]octane (85) resulted in an improvement of all measured physicochemical parameters: increased solubility, enhanced metabolic stability, and reduced lipophilicity.
Biological activity
Finally, to answer a key question, - whether the 2-oxabicyclo[2.2.2]octane core could indeed mimic the phenyl ring in bioactive compounds, we measured the biological activity of Imatinib versus its analogs 85, 86; and Vorinostat versus its analogs 88, 89.
We studied the inhibitory effect of Imatinib, Staurosporine, and compounds 85, 86 on the catalytic activity of ABL1 kinase. While the expected activity of Imatinib and Staurosporine was confirmed; we did not observe any significant inhibitory effect of compounds 85, 86 on the ABL1 kinase (please, see SI, pages 294, 295; Supplementary Figs. 13–15). The observed results correlate well with the previous study by Nicolaou, Vourloumis, and Stepan who demonstrated that the replacement of the para-substituted phenyl ring in Imatinib with various saturated cyclic cores, including bicyclo[1.1.1]pentane and cubane, led to a dramatic loss of potency against the ABL1 kinase67.
To study the biological activity of Vorinostat and its analogs 88, 89, we evaluated their effect on human hepatocellular carcinoma cells HepG2 by fluorescent microscopy (please, see SI, pages 296-300; Supplementary Figs. 16–19). The cells were incubated with the compounds for 48 hours. Staining with specific dyes revealed that all three compounds promoted caspase-dependent cell death, - apoptosis, - that further precipitated in necrosis when the cellular membrane lost its integrity. Vorinostat treatment resulted in 7.2% and 12.2% of apoptotic cells upon incubation at concentrations 5 μΜ and 50 μΜ respectively (Fig. 9). Analogs 88 and 89 demonstrated similar efficacy only at 50 μΜ.
Types of HepG2 cell death (% of total cells) after treatment with Vorinostat and compounds 88, 89 (1 μM, 5 μM, and 50 μM) for 48 h. Red: necrotic cell death. Green: early apoptotic cell death. The data were presented as mean ± SEM (n = 3, independent wells for every of which approx. 2000 visualized cells were analyzed).* - indicates P < 0.05, ** - indicates P < 0.01, *** - P < 0.001 compared with the non-treated group in a two-tailed unpaired t-test with Welch correction on each row of data.
These primary biological results (Fig. 9) suggested that Vorinostat and both its analogs 88, 89 could have similar cytotoxic and cytostatic activities in cells (for a more comprehensive comparison of Vorinostat and its analogs 88, 89, additional experiments on the enzyme potency and selectivity are needed).
Virtual libraries
To analyze how the replacement of the para-substitued phenyl ring with 2-oxabicyclo[2.2.2]octane affects 3D-shape of organic compounds, we generated two virtual libraries based on C- and N-terminus modifications of para-aminobenzoic acid and its 2-oxabicyclo[2.2.2]octane-containing analog. Each library contained 5000 molecules (Supplementary Data 6, Supplementary Data 7). According to principal moments of inertia (PMI) plots, both libraries occupied essentially the same region in 3D-chemical space. The same was true for FDA-approved drugs Aminopterin, Conivaptan, Deferasifox, Tetracaine, and their 2-oxabicyclo[2.2.2]octane-containing analogs (for details, please see SI, pages 301–304; Supplementary Table 8, Supplementary Figs. 20 and 21).
In conclusion, we have designed, synthesized, and characterized a new saturated bioisostere of the phenyl ring - 2-oxabicyclo[2.2.2]octane. In the design of the structure, we kept all advantages of the previously used cores (bicyclo[1.1.1]pentane, bicyclo[2.2.2]octane, cubane): conformational rigidity, metabolic stability, non-chirality, and collinearity of the exit vectors (Fig. 1c). In addition, we addressed their disadvantages: C-C distance and lipophilicity (Fig. 1c). Thus the 2-oxabicyclo[2.2.2]octane scaffold designed here was synthesized from available starting materials on a multigram scale (Table 1) - up to 135 g in one run (Fig. 2). The key synthesis step was the iodocyclization of cyclohexane-containing alkenyl alcohols with molecular iodine in acetonitrile (Figs. 2 and 3). Crystallographic analysis revealed its high similarity with the para-substituted phenyl ring (Fig. 5). 2-Oxabicyclo[2.2.2]octane core was incorporated into the structure of Imatinib and Vorinostat drugs instead of the para-substituted and the mono-substituted phenyl rings, correspondingly (Figs. 7 and 8). In the case of Imatinib, the formed saturated analog 85 possessed improved physicochemical properties over the drug: increased water solubility, enhanced metabolic stability, and reduced lipophilicity (Fig. 7). In the case of Vorinostat (SAHA), the formed saturated analog 88 exhibited a similar biological activity compared to that of the drug (Fig. 9).
This study enhances the repertoire of available saturated bioisosteres of (hetero)aromatic rings for use in drug discovery projects.
Methods
General procedure for the iodocyclization
To a solution of alkene 5 (222.64 g, 1.21 mol, 1.00 equiv) in MeCN (4000 mL) were added NaHCO3 (243.94 g, 2.90 mol, 2.40 equiv) in one portion and I2 (736.60 g, 2.90 mol, 2.40 equiv) in four portions. The resulting mixture was stirred for 12 h at room temperature. Then sodium thiosulfate pentahydrate (900.24 g, 3.63 mol, 3.00 equiv) and distilled water (2000 mL) were added to the mixture. The colorless mixture was extracted with MeOtBu (10 × 400 mL). The combined organic layers were concentrated under reduced pressure to dryness. The residue was dissolved in MeOtBu (1000 mL), washed with brine (1 × 400 mL), a saturated solution of Na2S2O3 (3 × 400 mL), dried over Na2SO4, filtered through a plug of SiO2 (0.5 L glass filter filed with 3 cm in high with silica gel) and concentrated. The final product was purified by column chromatography (SiO2, hexane:EtOAc = 1:5, Rf = 0.7) to provide pure iodide 6. Yield: 135.16 g, 0.436 mol, 36%, white solid.
NMR spectra were analyzed with MestreNova (11.0.3-18688).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Experimental data as well as characterization data for all new compounds prepared during these studies are provided in the Supplementary Information of this manuscript. The X-ray crystallographic coordinates for compounds 30, 57, 67, 69, and 78 have been deposited at the Cambridge Crystallographic Data Centre (CCDC) with accession codes 2226162 (30), 2226164 (57), 2226872 (67), 2226163 (69), 2266656 (78). These data can be obtained free of charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures/. A source data file is available for the biological activity of Imatinib with analogs 85, 86; and Vorinostat with analogs 88, 89. Source data are provided with this paper.
References
Chen, Y., Rosenkranz, C., Hirte, S. & Kirchmair, J. Ring systems in natural products: structural diversity, physicochemical properties, and coverage by synthetic compounds. Nat. Prod. Rep. 39, 1544–1556 (2022).
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Shearer, J., Castro, J. L., Lawson, A. D. G., MacCoss, M. & Taylor, R. D. Rings in clinical trials and drugs: present and future. J. Med. Chem. 65, 8699–8712 (2022).
The search was performed at https://go.drugbank.com on 05 July 2023.
Ritchie, T. J. & Macdonald, S. J. F. The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design? Drug Discov. Today 14, 1011–1020 (2009).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Measom, N. D. et al. Investigation of a bicyclo[1.1.1]pentane as a phenyl replacement within an LpPLA2 inhibitor. ACS Med. Chem. Lett. 8, 43–48 (2017).
Goh, Y. L., Cui, Y. T., Pendharkar, V. & Adsool, V. A. Toward resolving the resveratrol conundrum: synthesis and in vivo pharmacokinetic evaluation of BCP–resveratrol. ACS Med. Chem. Lett. 8, 516–520 (2017).
Pu, Q. et al. Discovery of potent and orally available bicyclo[1.1.1]pentane-derived indoleamine-2,3-dioxygenase 1 (IDO1) inhibitors. ACS Med. Chem. Lett. 11, 1548–1554 (2020).
Chalmers, B. A. et al. Validating eaton’s hypothesis: cubane as a benzene bioisostere. Angew. Chem. Int. Ed. 55, 3580–3585 (2016).
Wiesenfeldt, M. P. et al. General access to cubanes as benzene bioisosteres. Nature 618, 513–518 (2023).
Reekie, T. A., Williams, C. M., Rendina, L. M. & Kassiou, M. Cubanes in medicinal chemistry. J. Med. Chem. 62, 1078–1095 (2019).
Bernhard, S. S. R. et al. Cross-coupling and cubane–porphyrin arrays. Chem. Eur. J. 24, 1026–1030 (2018).
Houston, S. D. et al. The cubane paradigm in bioactive molecule discovery: further scope, limitations and the cyclooctatetraene complement. Org. Biomol. Chem. 17, 6790–6798 (2019).
Flanagan, K. J., Bernhard, S. S. R., Plunkett, S. & Senge, M. O. Not your usual bioisostere: solid state study of 3d interactions in cubanes. Chem. Eur. J. 25, 6941–6954 (2019).
Wlochal, J., Davies, R. D. M. & Burton, J. Cubanes in medicinal chemistry: synthesis of functionalized building blocks. Org. Lett. 16, 4094–4097 (2014).
Dallaston, M. A., Brusnahan, J. S., Wall, C. & Williams, C. M. Thermal and sensitiveness determination of cubanes: towards cubane-based fuels for infrared countermeasures. Chem. Eur. J. 25, 8344–8352 (2019).
Zhong, M. et al. Discovery of functionalized bisimidazoles bearing cyclic aliphatic-phenyl motifs as HCV NS5A inhibitors. Bioorg. Med. Chem. Lett. 24, 5731–5737 (2014).
Bandak, D., Babii, O., Vasiuta, R., Komarov, I. V. & Mykhailiuk, P. K. Design and synthesis of novel 19F-amino acid: a promising 19F NMR label for peptide studies. Org. Lett. 17, 226–229 (2015).
Aguilar, A. et al. Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): a potent and orally active murine double minute 2 (MDM2) inhibitor in clinical development. J. Med. Chem. 60, 2819–2839 (2017).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Locke, G. M., Bernhard, S. S. R. & Senge, M. O. Nonconjugated hydrocarbons as rigid-linear motifs: isosteres for material sciences and bioorganic and medicinal chemistry. Chem. Eur. J. 25, 4590–4647 (2019).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: Long–sought-after mimetics for ortho/meta-substituted arenes. PNAS 18, e2108881118 (2020). Replacement of the ortho- and meta-substituted phenyl rings in bioactive compounds with saturated bioisosteres was also recently achieved (Refs 26-31).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Epplin, R. C. et al. [2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes. Nat. Commun. 13, 6056 (2022).
Denisenko, A. et al. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 15, 1155–1163 (2023).
Dibchak, D. et al. General synthesis of 3-azabicyclo[3.1.1]heptanes and evaluation of their properties as saturated isosteres. Angew. Chem. Int. Ed. e202304246 (2023).
Kanazawa, J. & Uchiyama, M. Recent advances in the synthetic chemistry of bicyclo[1.1.1]pentane. Synlett 30, 1–11 (2019).
Ma, X. & Pham, L. N. Selected topics in the syntheses of bicyclo[1.1.1]pentane (BCP) analogues. Asian J. Org. Chem. 9, 8–22 (2020).
Gianatassio, R. et al. Strain release amination. Science 351, 241–246 (2016).
Caputo, D. F. J. et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 9, 5295–5390 (2018).
Pickford, H. D. et al. Twofold radical-based synthesis of N,C-difunctionalized bicyclo[1.1.1]pentanes. J. Am. Chem. Soc. 143, 9729–9736 (2021).
Bychek, R. & Mykhailiuk, P. K. A practical and scalable approach to fluoro-substituted bicyclo[1.1.1]pentanes. Angew. Chem. Int. Ed. 61, e202205103 (2022).
Pickford, H. D. et al. Rapid and scalable halosulfonylation of strain-release reagents. Angew. Chem. Int. Ed. 62, e202213508 (2023).
Ripenko, V., Vysochyn, D., Klymov, I., Zhersh, S. & Mykhailiuk, P. K. Large-scale synthesis and modifications of bicyclo[1.1.1]pentane-1,3-dicarboxylic acid (BCP). J. Org. Chem. 86, 14061–14068 (2021).
Bychek, R. M. et al. Difluoro-substituted bicyclo[1.1.1]pentanes for medicinal chemistry: design, synthesis, and characterization. J. Org. Chem. 84, 15106–15117 (2019).
Auberson, Y. P. et al. Improving non-specific binding and solubility: bicycloalkyls and cubanes as p-phenyl bioisosteres. ChemMedChem 12, 590–598 (2017).
Houston, S. D. et al. Cyclooctatetraenes through valence isomerization of cubanes: scope and limitations. Chem. Eur. J. 25, 2735–2739 (2019).
Takebe, H. & Matsubara, S. Catalytic asymmetric synthesis of 2,6-disubstituted cuneanes through enantioselective constitutional isomerization of 1,4-disubstituted cubanes. Eur. J. Org. Chem. 37, e202200567 (2022).
Wang, L. et al. Mechanochemistry of cubane. J. Am. Chem. Soc. 144, 22865–22869 (2022).
Toriyama, F. et al. Redox-active esters in Fe-catalyzed C–C coupling. J. Am. Chem. Soc. 138, 11132–11135 (2016).
He, J. et al. Ligand-promoted borylation of C(sp3)-H bonds with palladium(II). Catalysts. Angew. Chem. Int. Ed. 55, 785–789 (2016).
Hanson, A. M. et al. A−C estrogens as potent and selective estrogen receptor-beta agonists (SERBAs) to enhance memory consolidation under low-estrogen conditions. J. Med. Chem. 61, 4720–4738 (2018).
Shaw, S. A. et al. Discovery and structure activity relationships of 7-benzyl triazolopyridines as stable, selective, and reversible inhibitors of myeloperoxidase. Bioorg. Med. Chem. 28, 115723 (2020).
Koko, M., Anderluh, M., Hrast, M. & Minovski, N. The structural features of novel bacterial topoisomerase inhibitors that define their activity on topoisomerase IV. J. Med. Chem. 65, 6431–6440 (2022).
Singh, S. B. et al. Oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad spectrum antibacterial agents. ACS Med. Chem. Lett. 5, 609–614 (2014).
Singh, S. B. et al. Structure activity relationship of C-2 ether substituted 1,5-naphthyridine analogs of oxabicyclooctane-linked novel bacterial topoisomerase inhibitors as broad-spectrum antibacterial agents (Part-5). Bioorg. Med. Chem. Lett. 25, 3630–3635 (2015).
Singh, S. B. et al. C1–C2-linker substituted 1,5-naphthyridine analogues of oxabicyclooctane-linked NBTIs as broad-spectrum antibacterial agents (part 7). Med. Chem. Commun. 6, 1773–1780 (2015).
Tan, C. M. et al. In vitro and in vivo characterization of the novel oxabicyclooctane-linked bacterial topoisomerase inhibitor AM-8722, a selective, potent inhibitor of bacterial DNA gyrase. Antimicrob. Agents Chemother. 22, 4830–4839 (2016).
Harrison, T. J. et al. Successful strategies for mitigation of a preclinical signal for phototoxicity in a DGAT1 inhibitor. ACS Med. Chem. Lett. 10, 1128–1133 (2019).
Lu, Z. et al. Identification of potent, selective and orally bioavailable phenyl ((R)-3-phenylpyrrolidin-3-yl)sulfone analogues as RORγt inverse agonists. Bioorg. Med. Chem. Lett. 29, 2265–2269 (2019).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Ed. 59, 7161–7167 (2020).
Kraus, G. A. & Thurston, J. Alkoxy radicals in organic synthesis. A novel approach to spiroketals. Tetrahedron Lett. 28, 4011–4014 (1987).
Qin, T. et al. Nickel-catalyzed barton decarboxylation and giese reactions: a practical take on classic transforms. Angew. Chem. Int. Ed. 56, 260–265 (2017).
Yang, Y. et al. Practical and modular construction of C(sp3)-rich alkyl boron compounds. J. Am. Chem. Soc. 143, 471–480 (2021).
Wang, J. et al. Cu-catalyzed decarboxylative borylation. ACS Catal. 8, 9537–9542 (2018).
Yao, S. et al. Photoredox-promoted alkyl radical addition/semipinacol rearrangement sequences of alkenylcyclobutanols: rapid access to cyclic ketones. Chem. Commun. 54, 8096–8099 (2018).
Urner, L. M. et al. Systematic variation of cyanobuta-1,3-dienes and expanded tetracyanoquinodimethane analogues as electron acceptors in photoactive, rigid porphyrin conjugates. Eur. J. Org. Chem. 2015, 91–108 (2015).
Bloomfield, A. J., Chaudhuri, S., Mercado, B. Q., Batista, V. S. & Crabtree, R. H. Facile solvolysis of a surprisingly twisted tertiary amide. N. J. Chem. 40, 1974–1981 (2016).
Nagar, B. et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and Imatinib (STI-571). Cancer Res. 62, 4236–4243 (2002).
Doerwald, F. Z. Lead optimizations for medicinal chemists (Wiley-VCH, 2012).
clogP was calculated with “Cxcalc” (ChemAxon, version 22.5.0) (2022).
Nicolaou, K. C. et al. Synthesis and biopharmaceutical evaluation of imatinib analogues featuring unusual structural motifs. ChemMedChem 11, 31–37 (2016).
Acknowledgements
The authors are grateful to Prof. A. A. Tolmachev for the support of this work. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 101000893 - BENOVELTY). P.K.M. is very grateful to Dr. S. Shishkina (IOC, Kyiv) for the X-ray studies, to Dr. D. Bylina for HRMS measurements, to K. Fominova and V. Kokhalskiy for the help with the synthesis of compounds 35 and 39, and to Dr. V. Kubyshkin for proofreading the manuscript.
Author information
Authors and Affiliations
Contributions
V.V.L., I.S., P.B., and P.K.M. designed the experiments. V.V.L., Y.P., K.S., O.S., V.B., O.S., I.S., L.B., O. K.-U., Y.H., L.L., P.B., K.H., I.B., J.P.B., D.D. conducted and analyzed the experiments described in this report. P.B. and P.K.M. prepared this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: V.V.L., Y.P., K.S., O.S., V.B., O.S., I.S., L.B., O.K.-U., Y.H., P.B., K.H., I.B., D.D., P.K.M. are employees of a chemical supplier Enamine. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Edward Anderson, Bin Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Levterov, V.V., Panasiuk, Y., Sahun, K. et al. 2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring. Nat Commun 14, 5608 (2023). https://doi.org/10.1038/s41467-023-41298-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41298-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.