Abstract
An efficient one-pot strategy for the facile synthesis of double boron–oxygen-fused polycyclic aromatic hydrocarbons (dBO-PAHs) with high regioselectivity and efficient skeletal editing is developed. The boron–oxygen-fused rings exhibit low aromaticity, endowing the polycyclic aromatic hydrocarbons with high chemical and thermal stabilities. The incorporation of the boron–oxygen units enables the polycyclic aromatic hydrocarbons to show single-component, low-temperature ultralong afterglow of up to 20 s. Moreover, the boron–oxygen-fused polycyclic aromatic hydrocarbons can also serve as ideal n-type host materials for high-brightness and high-efficiency deep-blue OLEDs; compared to single host, devices using boron–oxygen-fused polycyclic aromatic hydrocarbons-based co-hosts exhibit dramatically brightness and efficiency enhancements with significantly reduced efficiency roll-offs; device 9 demonstrates a high color-purity (Commission International de l’Eclairage CIEy = 0.104), and also achieves a record-high external quantum efficiency (28.0%) among Pt(II)-based deep-blue OLEDs with Commission International de l’Eclairage CIEy < 0.20; device 10 achieves a maximum brightnessof 27219 cd/m2 with a peak external quantum efficiency of 27.8%, which representes the record-high maximum brightness among Pt(II)-based deep-blue OLEDs. This work demonstrates the great potential of the double boron–oxygen-fused polycyclic aromatic hydrocarbons as ultralong afterglow and n-type host materials in optoelectronic applications.
Similar content being viewed by others
Introduction
Polycyclic aromatic hydrocarbons (PAHs), especially heteroatom-embedded PAHs, have attracted considerable attention1 because of their unique catalytic activity and photophysical/photoelectronic properties, enabling extensive and promising applications in catalysis2 and organic electronics, such as organic photovoltaics (OPVs)3, organic field-effect transistors (OFETs)4,5, organic phototransistors (OPTs)6, and organic light-emitting diodes (OLEDs)7,8,9,10,11. However, because of strong vibronic coupling and aromaticity, PAHs typically show broad structural emission spectra and low T1 energy levels (ET1) (Supplementary Fig. 1)9. Among the various main-group elements used as dopants, boron is particularly attractive, owing to its sp2 hybridization and the presence of the vacant pz orbital; these features enable the efficient regulation of the molecular skeletons and π-conjugation of the boron-embedded PAHs (B-PAHs), as well as the further tuning of their excited-state properties and charge-transfer characteristics, in order to develop a variety of novel functional materials1,7,8,9,10,11,12,13. In particular, boron/nitrogen-embedded PAHs (BN-PAHs) with rational molecular design demonstrated extremely narrowband Gaussian-type emission spectra because of the minimized vibronic coupling by multiple resonance (MR) effect7,8,9. However, compared with the widely studied B-PAHs and BN-PAHs1,6,7,8,9,10,11,12,13,14,15,16,17, scarce data are available on PAHs containing B–O unit (BO-PAHs), and in particular on their optoelectronic properties, due to their inefficient synthetic methods.
Since Dewar and Dietz’s pioneering work on the synthesis of 9,10-boroxarophenanthrene in 196018, several groups made good progress on investigating the chemistry of BO heteroarenes in recent years (Fig. 1a)19,20,21,22,23. However, some issues still exist, such as harsh reaction conditions, separation of air-sensitive intermediates, or low isolated yields. Thus, the development of facile synthetic methods, which can facilitate the synthesis of chemically diverse BO-PAHs and the systematic investigation of their structure-property relationship, is still highly desired. These are fundamental and critical targets for both chemists and material scientists, whose achievement would also be beneficial to promote the applications of the BO-PAHs as functional materials.
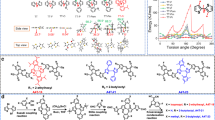
a Previous reported BO-PAHs. b Design concept of versatile skeletal editing and facile synthesis of dBO-PAHs through one-pot strategy developed in this work. The versatile skeletal editing can be easily realized by Ar1, Ar2 or OMe position regulation; the arrows indicate the position of electrophilic borylation directed by the OMe group. Ph, phenyl group, Mes, 2,4,6-trimethylphenyl group, Ar1, Ar2, aryl group, R, R1, R2, substituent group.
Herein, we report an efficient method for the facile fabrication of doubly BO-fused PAHs (dBO-PAHs) with versatile molecular skeletons via a one-pot strategy of tandem demethylation-electrophilic borylation-nucleophilic substitution reactions (Fig. 1b). The molecular cores of the dBO-PAHs could be efficiently edited with high regioselectivity by regulating the positions of the aryl (Ar1 and Ar2) and methoxyl (OMe) groups in the phenyl rings of the substrates; the aryl and OMe moieties acted as guiding and directing groups, respectively. Using this method, doubly BO-fused central symmetric pyrenes (Type I) and benzo[m]tetraphenes (Type III and Type VI), C2-symmetric benzo[k]tetraphenes (Type IV and Type V), and asymmetric benzo[c]chrysene (Type II) were successfully synthesized (Fig. 1b). Their molecular geometries, photophysical and thermal properties were systematically investigated through crystallographic studies, optical spectroscopy, and thermal gravimetric analysis (TGA). Theoretical and experimental studies revealed that the incorporation of the B–O units into the PAHs greatly affected their aromaticity and electronic properties. The synergistic effects of the p-π conjugation, the delocalization of the lone electron pairs of the oxygen atoms as well as the bulky Mes moieties (Mes: 2,4,6-trimethylphenyl) enabled the dBO-PAHs to possess good stabilities. The dBO-PAHs showed strong fluorescence emission with quantum efficiencies (ΦPL) of up to 95% at room temperature (RT) in dichloromethane; moreover, most dBO-PAHs exhibit single-component, dual-emission with both nanosecond fluorescence and second-level phosphorescence at 77 K in 2-methyltetrahydrofuran (2-MeTHF), and the persistent phosphorescence enabled the dBO-PAHs to exhibit an ultralong low-temperature afterglow of up to 20 s after UV excitation. Furthermore, some dBO-PAHs could also serve as ideal n-type host materials for high-brightness and high-efficiency deep-blue OLEDs; compared to single host, BO1b-based co-host systems enabled deep-blue OLEDs to exhibit dramatic brightness and efficiency enhancements with significantly reduced efficiency roll-offs; PtON1 and PtON-TBBI-based OLEDs realized a record-high maximum brightness (Lmax, 27219 cd/m2) and a record-high external quantum efficiency (EQE, 28.0%) among Pt(II)-based deep-blue OLEDs with Commission International de l’Eclairage (CIE) y < 0.20, respectively.
Results
Synthetic method development and structure characterization
To develop a facile synthetic method for dBO-PAHs, we envision a one-pot strategy to avoid the separation of the air-sensitive intermediates (Int 1 and Int 3) via tandem demethylation-electrophilic borylation-nucleophilic substitution reactions using dimethoxybiaryls or dimethoxytriaryls as starting materials (Fig. 2). However, the simultaneously form two B–O bonds and four B–C bonds remains challenging because of the complex reaction system involved; in particular, in the synthesis of dBO-PAHs with two boron atoms in the same phenyl ring, e.g., Type I, Type V, and Type VI compounds, it is difficult to attain the second electrophilic borylation on an electron-deficient phenyl ring (Fig. 2).
After optimizing the reaction conditions (see Supplementary Fig. 2 for details), it was found that pure BBr3 could accelerate the demethylation; additionally, nonpolar solvents improved the solubility of the intermediates and enhanced the reaction rate. Finally, a 94% isolated yield of BO1a in gram scale could be achieved under mild conditions using hexane/toluene as a solvent in the presence of 2.5 equiv of pure BBr3, 4–8 mol% AlCl3 and 5.0 equiv of MesMgBr. Notably, the bulky Mes groups were incorporated to enhance the chemical stability by protecting the boron atoms from nucleophilic reagents24.
As illustrated in Fig. 3, the one-pot protocol exhibited a broad substrate scope. For the synthesis of central symmetric double BO-fused pyrenes (Type I), electron-rich alkyl and aryl substitutions on either of the phenyl rings were tolerated in moderate to excellent yields (68–94%), however, 2.0 equiv AlCl3 was needed for BO1e to promote the complete conversion of intermediate. Interestingly, upon adjusting the position of one phenyl group (Ar2) from 1,3-dimethyl-2,5-diphenylbenzene (A1f) to 1,3-dimethyl-2,4-diphenylbenzene (A2), the asymmetric dBO-benzo[c]chrysene BO2 (Type II), bearing two boron atoms on different phenyl rings, could be exclusively separated in 80% yield, and the corresponding Type I compound was not observed; this revealed that the first borylation was dominated by the steric effect, and the second electrophilic borylation had a high regioselectivity and preferentially occured on an electron-rich phenyl (Fig. 1b). By further adjusting the Ar1 position of A2, another series of dimethoxybiaryls A3 were designed, which generated dBO-benzo[m]tetraphenes (Type III) with central symmetric skeletons (Fig. 1b). Notably, this reaction system also tolerated β-naphthyl and 2-fluorenyl groups, forming the extended π-conjugation molecules BO3d and BO3e with polygonal-shaped skeletons in 79% and 41% yields, respectively (Fig. 3). On the other hand, by adjusting both OMe and Ar2 positions of A2, the obtained A4 smoothly generated C2-symmetric Type IV BO4a–BO4e in 43–85% yields, in which methyl, sterically hindered α-naphthyl, β-naphthyl, and 2-fluorenyl groups were all tolerated (Figs. 1b, 3). Unexpectedly, the electrophilic borylations exclusively occurred on the relatively low-reactivity β-positions of the naphthyl groups in BO4d (Fig. 3), revealing that steric effects prevailed over electronic effects. Importantly, the positions of the boron atoms in the molecular skeletons could be effectively edited by simply adjusting the OMe position of A4 and A3 to produce the corresponding C2-symmetric BO5 (Type V) and central symmetric BO6 (Type VI) with high regioselectivities in 83% and 81% yields, respectively (Figs. 1b, 3); the regioselectivities were attributed to the steric effects of the Mes groups, which impeded the last nucleophilic substitution reaction to form isomers. Notably, all attempts to introduce tert-butyl into the Type I and Type III BO-PAHs failed, and only complete de-tert-butylation products of retro-Friedel–Crafts reactions were isolated25 owing to the strong Lewis acidity of AlCl3 (Supplementary Fig. 3). Ortho-analogs were also tried to synthesized by using the precursors of 1,4-dibromo-2,3-dimethoxy-5,6-dimethylbenzene (A7a) and 2,3-dimethoxy-1,4-diphenylnaphthalene (A7b), but they gave messy results (see supporting information for details). The dBO-PAHs were highly stable and could be easily purified through column chromatography on silica gel. Importantly, they were also insensitive to air and moisture, and no degradation was observed after keeping them in the solid state under ambient conditions for more than 1 year. All the newly developed dBO-PAHs were characterized by 1H, 13C, and 11B nuclear magnetic resonance (NMR) spectroscopy and high-resolution mass spectrometry (HRMS), and the results were consistent with the proposed molecular structures. Importantly, large-scale synthesis of BO1b, BO1c, BO2, and BO3a were also carried out for their further device fabrication as host materials; they all could be smoothly obtained using 4 mol % AlCl3 with isolated yields of 80% (9.56 g), 87% (16.30 g), 80% (6.71 g) and 82% (10.98 g), respectively. The developed method can provide versatile dBO-PAHs, enabling the investigation of their potential applications in organic electronics.
The dBO-PAHs synthesized via one-pot protocol with 4 or 8 mol% AlCl3 was used. The single-crystal X-ray structures are shown with thermal ellipsoids at 50% probability, and hydrogen atoms are omitted for clarity. NICS(1) values calculated with a B3LYP/6-31 G(d) basis set in the gas phase are shown. The obtained product weight was provided in parentheses. a20 mol% AlCl3 was used. bA by-product 6-mesityl-8-methyl-2-(p-tolyl)−6H-dibenzo[c,e][1,2]oxaborinin-3-ol (BO3b-OH, CCDC: 2290997) was also isolated in 41% yield. c2.0 equiv AlCl3 was used.
Crystallographic analyses
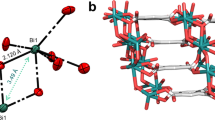
To further confirm the molecular structures of the dBO-PAHs and determine their molecular geometries, single crystals of BO1a, BO2, BO3a, BO3d, BO4b, BO4d, BO4e, BO5, and BO6 were carefully grown and analyzed by X-ray diffraction. The crystal structures, selected bond lengths, bond angles, dihedral angles, and solid-state packing structures are illustrated in Fig. 3, Fig. 4, and Supplementary Figs. 4–14, and the crystal data are listed in Supplementary Tables 1–4 and Supplementary Data 1–10. The crystal diffraction analyses clearly support the molecular structures obtained from NMR and HRMS data (Fig. 3). All the dBO-PAHs had almost planar BO-fused cores, supported by the fact that the sums of the one C–B–C and two C–B–O bond angles around the boron atoms were almost 360.0° for all the dBO-PAHs, revealing the sp2 hybridization of the central boron atoms. However, BO3a exhibited a slight bent core with a dihedral angle of 10.33° between the two terminal phenyl rings (Supplementary Fig. 8). Moreover, the B–C bond lengths of the BO-cores in BO1a, BO2, BO4b, BO4d, BO4e, BO5, and BO6 ranged between 1.522 and 1.549 Å; these values were significantly shorter than those of their B–CMes bond lengths (1.564–1.578 Å) and the reported B–C bond lengths of Mes3B (1.573–1.580 Å)26. The short B–O bond lengths are from 1.368 Å to 1.384 Å, which are in line with previous reports19,21,22. These results reveal that the empty boron pz orbitals were stabilized not only by the strong p-π conjugation with adjacent phenyl rings, but also by the delocalization effect of the lone electron pairs from the directly linked oxygen atoms, endowing the dBO-PAHs with excellent stabilities. The p-π conjugation and the delocalization effect were also supported by the 11B NMR spectra of the dBO-PAHs, which showed significant upfield shifts (δ = 44.68–49.59 ppm) compared to that of the Mes3B (δ = 79.0 ppm)27. These results reveal the importance of the enforced planarity of the BO cores for the fabrication of stable dBO-PAHs. Furthermore, as expected, the BO cores were almost spatially perpendicular to the bulky Mes groups, enabling the boron atoms to be shielded by the methyl groups in the ortho position of the Mes moieties (Supplementary Fig. 5), which was also beneficial for the stability enhancement.
All the dBO-PAHs had monoclinic crystal systems, except BO2 and BO4b, which crystallized in orthorhombic systems (Supplementary Tables 1–4). Continuous π-stacks with varying degrees of slipped stacking between the BO cores were observed in all dBO-PAHs except BO6 (Fig. 4, and Supplementary Figs. 6–13). BO3a and BO4b showed small interplanar distances of 3.446 and 3.685 Å, respectively, indicative of strong intermolecular π···π interactions (Fig. 4, and Supplementary Figs. 8, 10). In contrast, BO2, BO3d, and BO4e exhibited large interplanar distances of 5.967, 6.404, and 5.536 Å, respectively (Supplementary Figs. 7, 9, 12). Notably, BO1a showed two types of π-stacks in almost perpendicular directions, with interplanar distances of 3.619 and 6.092 Å (Fig. 4, and Supplementary Fig. 6). On the other hand, intermolecular C–H···π interactions between the Mes groups were also observed for BO3a, BO4b, and BO4d with distances of 3.183, 2.859, and 2.883 Å, respectively (Supplementary Figs. 8, 10, 11). Nevertheless, BO3d exhibited intermolecular C–H···π interactions between the BO cores, with short distances of 2.722–2.845 Å (Supplementary Fig. 9), and C–H···π interactions between fluorenyl moieties in BO4e (Supplementary Fig. 12) and between Mes and BO core in BO5 (Supplementary Fig. 13) were also observed. Most interestingly, BO6 showed solid-state stacking with perpendicularly overlapping head–tail configurations with intermolecular C–H···π distances of 3.049–3.372 Å (Fig. 4, and Supplementary Fig. 14). These results reveal that both the BO cores and Mes groups considerably affected the solid-state structures. The ubiquitous π-stacks are beneficial for charge carrier transfer in organic electronic applications6,28.
Theoretical analyses
To further understand the aromaticity and electronic properties of the dBO-PAHs, we performed density functional theory (DFT) and nucleus-independent chemical shift (NICS) calculations (Fig. 3, and Supplementary Figs. 15–19). The BO-fused rings typically had NICS(1) values from –2.1 to –2.8, which were comparable to that of the previously reported BN-fused dibenzo[g,p]chrysene (–2.9)16, suggesting a low aromaticity. Additionally, all the neighboring phenyl rings showed much more negative NICS(1) values, similar to their corresponding PAH analogs, e.g., BO1a (–11.58 and –11.57) vs. CC1a (–13.71 to –13.64) (Supplementary Fig. 15), revealing that the BO moieties hardly affected the aromaticity of the surrounding phenyl rings. Both the above effects enhance the stability of the dBO-PAHs.
The DFT calculations showed that the dBO-PAHs had similar lowest unoccupied molecular orbital (LUMO) levels to their corresponding carbon analogs; however, they possessed remarkably stabilized highest occupied molecular orbitals (HOMOs), resulting in significantly large HOMO–LUMO energy gaps (Eg), this was attributed to the delocalization effect for BO1 series and BO6, and the electron-deficient property of the boron for BO2, BO3 series, BO4 series and BO5 (Supplementary Figs. 16–19); for instance, the LUMO, HOMO, and Eg values were –1.47, –5.28, and 3.81 eV for CC1a vs. –1.50, –6.06, and 4.56 eV for BO1a, respectively (Supplementary Table 5). As illustrated in Supplementary Figs. 16–19, the LUMOs of all dBO-PAHs except BO4d showed a nonuniform distribution on the whole BO cores, and were predominantly located on the boron atoms and the connected benzene rings. The HOMOs of BO2, BO3a, BO4a, and BO5 fully distributed on the whole BO cores, whereas they were predominantly on the Mes groups for BO1a and BO6 (Supplementary Fig. 17). The fused phenyl groups (BO3d, BO4c, BO4d) and incorporated fluorenyls (BO3e, BO4e) significantly extended both the HOMO and LUMO distributions (Supplementary Figs. 18, 19). The substituent phenyl groups in BO3c extended the LUMO distribution, but hardly affected the HOMO distribution (Supplementary Fig. 18); however, the completely opposite effect was observed for BO1d (Supplementary Fig. 16). These results indicate that the skeletal editing, substituent modification, and tuning of the π-conjugation had great influence on the electronic structures of the dBO-PAHs, and could be also used to regulate their energy levels and gaps.
Photophysical properties
The photophysical properties of the boron-containing PAHs are important for their applications in organic electronics. Thus, their absorption and emission properties were systematically investigated and illustrated in Fig. 5 and Supplementary Figs. 20–42, whereas the corresponding data were listed in Table 1. The strong absorption bands of the dBO-PAHs below 310 nm were assigned to π–π* transitions. The bands in the low-energy region, typically > 325 nm, were identified as intramolecular charge transfer (ICT) transitions introduced by the electron-deficient BO moieties. Introducing electron-donating group (Me) or extending the π-conjugation gradually red-shifted the highest-wavelength absorption band, e.g., 354, 357, 397, 376, and 385 nm for BO4a, BO4b, BO4c, BO4d, and BO4e, respectively, and the same effect was also observed for the BO3 series (Fig. 5, Supplementary Fig. 20). The fused π-conjugation from the C–C bond at the β-position of the naphthyl moiety dramatically increased the absorption intensities of both π–π* and ICT transitions, e.g., ε = 5.57 × 104 (262 nm) and 3.47 × 104 (290 nm) for BO3a vs. ε = 12.98 × 104 (284 nm) and 4.87 × 104 (370 nm) for BO3d, as well as ε = 2.26 × 104 (307 nm) and 2.95 × 104 (354 nm) for BO4a vs. ε = 8.52 × 104 (295 nm) and 5.21 × 104 (376 nm) for BO4d (Table 1, Fig. 5, Supplementary Fig. 20). Additionally, the experimentally calculated oscillator strength (f) of S1-S0 for BO4e was estimated to be 0.24 (see supporting information for details), the high f was attributed to the C2 symmetry of the molecular geometry, and extended linear conjugation system by two fluorenyl units, as well as the incorporation of the BO units, which could induce a larger transition dipole moment compared to no BO-fused PAHs29,30.
Excitingly, all the dBO-PAHs showed strong emission in dichloromethane at room temperature, peaking at 355–414 nm with relatively small full width at half-maximum (FWHM) values of 25–57 nm (Table 1, Supplementary Figs. 21, 23–42). Most dBO-PAHs exhibited well-resolved vibronic emission spectra, and dBO-PAHs with large π-conjugation showed one dominant emission peak with small FWHM value, like BO3d, BO3e and BO4e; by contrast, BO1f, BO1g and BO2 displayed unstructured and broad emission spectra, resulting in large FWHM values, this should be attributed to their relatively strong electronic vibronic coupling between the S0 and excited states as well as by structural relaxation at the excited states. The dBO-PAHs also possessed ΦPL values of 23–95%, with short excited state lifetimes (τf) of 1.2–5.8 ns, resulting in large radiative rates (kr) typically at the order of 108 (Table 1). BO1 series, BO2 and BO6 had low quantum efficiencies due to their small kr and large nonradiative rate (knr); BO3, BO4 series and BO5 showed high quantum efficiencies because of the enhanced radiative rate kr. In particular, BO4a and BO4b had relatively low ΦPL of 62% and 50%, respectively; BO4e showed an ultra high ΦPL of 95%, together with a short τf of 1.4 ns, leading to a large kr of 6.8 × 108 s−1 and a small knr of only 0.4 × 108 s−1 (Table 1). The high ΦPL of BO4e was attributed to the ICT characteristics, reflecting the importance of the BO incorporation; and also BO4e had larger f (0.24) than those of BO4a (f = 0.092) and BO4b (f = 0.12) (see supporting information for details). These results suggest the potential application of the present compounds as near-ultraviolet or deep-blue emitters in OLEDs, especially for the BO3 and BO4 series with high ΦPL (Table 1).
Ultralong low-temperature phosphorescence
Single-component, dual-emissive materials are highly desirable because of their potential applications in bio-imaging, ratiometric/optical sensing, and oxygen detection31,32,33. Ultralong organic phosphorescence (UOP) materials also have wide and promising applications in the areas of surface icing indications, optical thermometry, biological labeling, and virus preservation in extreme temperature conditions34,35,36,37. Traditionally, the UOP materials have been extensively developed via host-guest doping, crystallization, polymerization, etc.;38 however, single-component UOP materials with long second-level phosphorescence lifetimes (τp) are rare39,40,41. At 77 K in 2-MeTHF, most of the dBO-PAHs, especially the BO1 series, BO2, BO3a–BO3d, BO4a, BO4b, BO5, and BO6, exhibited prominent single-component dual emissions with nanosecond fluorescence (τf = 1.2–12.6 ns) and UOP (τp = 0.3–5.0 s) (Table 1, Fig. 6, Supplementary Figs. 23–44).
a–f Comparison of room-temperature PL spectra in dichloromethane, as well as 77 K PL and phosphorescent spectra in 2-MeTHF of BO1a, BO1d, BO1e, BO4a, BO5, and BO6, along with corresponding ultralong organic phosphorescence (UOP) photographs at 77 K in THF; the corresponding 77 K phosphorescent lifetime (τP) is shown in the inset. g Comparison of PL decay curves of BO1d, BO2, BO3a, BO4a, BO5, and BO6 at 77 K in 2-MeTHF upon excitation at their corresponding first phosphorescent peak. h, i TD-DFT-calculated singlet and triplet energy levels, and main transition configurations of BO1a and BO6 obtained at the B3LYP/6-31 G(d) level based on the optimized S0 geometry; as well as spin-orbit coupling (SOC) matrix elements between S1 and Tn states of BO1a and BO6 evaluated using PySOC at the B3LYP/6-31 G(d,p) level. RT room temperature, PL photoluminescence spectrum, Phos phosphorescence spectrum, THF tetrahydrofuran, 2-MeTHF 2-methyltetrahydrofuran, ISC intersystem crossing, IC internal conversion, ξ SOC value, ΔE energy gap, H HOMO, L LUMO.
As shown in Fig. 6a, BO1a showed dual emission at 77 K, in which the 330–400 nm fluorescent component with τf 3.6 ns was in good agreement with the RT spectrum with dominant peaks at 348 and 362 nm and τf 2.4 ns; a much stronger 400–550 nm phosphorescent component could be selectively recorded by time-resolved PL spectroscopy, and was also in line with the steady-state PL spectrum at 77 K. Similar low-temperature phosphorescence spectra could be also recorded for BO1d, BO1e, BO4a, BO5 and BO6, whose fluorescent components completely disappeared because of their short lifetimes (Fig. 6b–f ). Additionally, the ratios of the fluorescence and phosphorescence intensities for the dBO-PAHs were strongly dependent on the nature of the double BO cores and the electronic effect of the substituents on the cores; taking the BO1 series as an example, the incorporation of electron-donating groups into the core or the extension of the conjugation system of the core resulted in a decreased phosphorescence intensity (Fig. 6, Supplementary Figs. 23–42). Importantly, the long second-level τp (Fig. 6g, Supplementary Fig. 44) enabled the dBO-PAHs to exhibit strong and ultralong low-temperature afterglow of up to 20 s after turning off the UV light source (Fig. 6a–f, Supplementary Figs. 45, 46). Moreover, the dBO-PAHs also exhibited distinct color changes for the low-temperature UOP after switching the UV light off, e.g., deep blue vs. greenish blue for BO1d and BO1e, deep blue vs. sky blue for BO2, BO3b, BO3c and BO6, deep blue vs. green for BO4a and BO5, and blue vs. yellow for BO4c and BO4d (Fig. 6a–f, Supplementary Figs. 45, 46); this highlighted their potential application in anti-counterfeiting and information encryption40,42. Notably, no UOP property was observed for the dBO-PAHs in PMMA films at room temperature or the ice point.
Typically, metal-free organic molecules lack a sufficient intersystem crossing (ISC) from their singlet to triplet states and thus exhibit a rapid radiative decay to the ground states, resulting in no or very weak phosphorescence. Therefore, halogens or carbonyls need to be introduced into the phosphorescent organic molecules to facilitate the spin-orbital coupling (SOC) and further enhance the ISC rate;38 however, these strategies enhance the rates of both Sn → Tn and Tn → S0 transitions, resulting in shorter phosphorescent lifetimes41. Thus, the design and development of low-temperature UOP is still a challenge, because this is required to accelerate the ISC from S1 to triplet state and simultaneously decelerate the radiative decay from T1 to ground state, achieving to accumulate enough triplet excitons. To obtain a deep understanding of the UOP properties of the dBO-PAHs, TD-DFT calculations were performed on their excited state levels and some main transition configurations (Fig. 6h, i, Supplementary Figs. 47–51). As illustrated in Fig. 6h, BO1a had an extremely small S1-T8 energy gap (ΔES1-T8) of –0.023 eV, along with the same transition orbital composition of 97% HOMO → LUMO for S1 and 73% HOMO → LUMO for T8, facilitating an efficient ISC from S1 to T8; TD-DFT calculations also showed that SOC values of ξ(S1-T7) and ξ(S1-T5) were very large, 1.01 and 1.61 cm-1, respectively, together with small ΔEST values of 0.185 and 0.052 eV, revealing that the ISC from S1 to T7 and T5 were also efficient pathways according the Fermi’s golden rule. Moreover, natural transition orbital (NTO) analyses indicated that ISC from S1 to T8 was complete local excited (LE) character, and ISC from S1 to T7 had hybridized local and charge transfer (HLCT) characters in the molecular core (Supplementary Fig. 47). Then, the internal conversion (IC) from T8, T7, T5, to T1 can occur rapidly, due to several small energy gaps separating them. In addition, the large ΔES1-T1 of 0.386 eV and the mismatched transition orbital compositions of T1 and S1 prevent the reverse ISC from T1 to S1. Moreover, the rigid molecular geometry of BO1a (Supplementary Fig. 4) and the surrounding solid environment suppress the nonradiative decay. All the above factors endow BO1a with a long τp (3.1 s). Similarly, BO6 exhibited an even smaller ΔES1-T2 of only 0.013 eV, the same transition orbital composition for S1 (99% HOMO → LUMO) and T2 (97% HOMO → LUMO) and large ξ(S1-T2) of 0.93 cm-1 (Fig. 6i); the ISC from S1 to T2 also showed complete LE character in the molecular core (Supplementary Fig. 47). In contrast, BO1d had small ΔES1-T7 of 0.021 eV, but the ξ(S1-T7) was very tiny (0.24 cm−1), this resulted in slow ISC and relatively long UOP (Supplementary Fig. 47). These results provide a new perspective for the molecular design and development of metal- and halogen-free, single-component, dual-emission materials with UOP.
Thermal stability
Besides the chemical stability discussed above, the thermal stability of the doubly BO-PAHs is also important for their applications in organic electronics43,44. TGA measurements showed that BO1b, BO2, BO4a, BO5, and BO6 exhibited decomposition temperatures at 5% weight loss (ΔT5%) of 297, 343, 387, 339, and 335 °C, respectively (Supplementary Fig. 52). The good thermal stabilities can be attributed to the high dissociation energy of the B–O bond (536 kJ/mol)45. Additionally, no glass transition temperature was observed in the corresponding differential scanning calorimetry (DSC) measurements (Supplementary Fig. 52), indicating good morphological stability.
Performance of dBO-PAHs as host materials for high-brightness deep-blue OLEDs
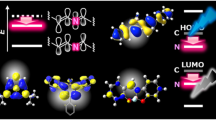
OLEDs are advanced devices with applications in full-color displays and solid-state lighting; efficient and high-brightness deep-blue phosphorescent OLEDs (POLEDs) with Commission International de l’Eclairage (CIE) y coordinate (CIEy) below 0.20 are essential for outdoor application46. However, deep-blue POLEDs typically exhibit a severe efficiency decrease at high luminescence47,48,49, known as efficiency roll-off. Because of the high ET1 of the deep-blue phosphorescent emitters (2.70–2.75 eV), very few host materials can be selected to solve this problem; therefore, novel host materials with high ET1 (above 2.80 eV) are highly needed50. As shown in Fig. 7a, compared to CC1b, BO incorporation greatly affected the electronic structure of BO1b, which exhibited a delocalized HOMO distribution over the whole molecule (that is, not only on the BO cores, but also on the Mes groups), resulting in a stabilized HOMO level and a larger Eg. Similar results were also observed for BO1e and BO1g (Supplementary Fig. 16, Supplementary Tables 5, 6). Additionally, BO1b exhibited a perfect reversible reduction process in the cyclic voltammetry (CV) measurement (Supplementary Fig. 53). Moreover, because of the weakened π-conjugation of the BO core, BO1b had a significantly higher calculated ET1 (3.10 eV) than CC1b (2.11 eV), which was also in good agreement with the experimental value of 3.00 eV (Table 1). The large Eg, high ET1, together with the high thermal stability of dBO-PAHs enable them to serve as host materials for deep-blue OLEDs (Fig. 7b).
a Comparison of calculated frontier orbital distributions, energy levels/gaps, and NICS(1) values of BO1b and its carbon analog CC1b. b CIEy vs. maximum brightness for Pt(II) and Ir(III)-based deep-blue OLEDs with CIEy < 0.20; devices connected with broken line to represent the devices with the same blue emitter; maximum EQE (%), and EQEs (%) at 100 and 1000 cd/m2 were provided in parentheses for representative deep-blue OLEDs. see Table S7 and Table S8 for detailed information. c, Chemical structures of host materials and Pt(II)-based deep-blue emitters used in this study. d, g, j EL spectra of deep-blue OLEDs at 1000 cd/m2. e, h, k Current density–voltage–luminance plots. f, i, l EQE vs. luminance plots. Eg the energy gap between HOMO and LUMO, ET1 the energy level of the lowest triplet excited state, EL electroluminescence spectrum, EQE external quantum efficiency.
BO1b was investigated first. To demonstrate the potential of BO1b as host material, single carrier devices were fabricated (Supplementary Fig. 54); BO1b possessed a good electron-transporting ability, but hardly transported holes, suggesting that BO1b could serve as n-type host material. Then, deep-blue OLEDs were fabricated by vacuum deposition with a device architecture of ITO/HATCN (10 nm)/BPBPA (70 nm)/26mCDTPy (5 nm)/emitting layer (EML, 25 nm)/DPEPO (10 nm)/TmPyPB (30 nm)/Li2CO3 (1 nm)/Al, where the EML consisted of 8% PtON1:92% 26mCPy (device 1), 8% PtON1:92% mCP (device 2), 8% PtON1:92% mCBP (device 3), 8% PtON1:92% BO1b (device 4); PtON1 (ET1 = 2.82 eV)48 was used as blue emitter, and the traditional bipolar host 26mCPy (ET1 = 2.92 eV)46,51,52,53,54, p-type hosts mCP (ET1 = 2.95 eV)54 and mCBP (ET1 = 2.95 eV)50 were employed to compare the corresponding device performances (Fig. 7c, Supplementary Fig. 55, Table 2). All the four devices showed similar turn-on voltage, however, PtON1:BO1b device 4 exhibited a significantly narrow electroluminescence (EL) spectrum with FWHM of 50 nm, resulting in remarkable color purity improvement, and the CIEy value of device 4 (0.134) was much smaller than those of devices 1–3 (0.159–0.171) (Fig. 7d, e, Table 2). Importantly, compared with devices 1–3, device 4 also demonstrated enhanced peak external quantum efficiency (EQE) of 22.8%, which still retained high EQEs of 21.5% at 100 cd/m2, and 16.3% at 1000 cd/m2 (Fig. 7f, Table 2); but device 4 showed large efficiency roll-off in high brightness region of > 1000 cd/m2 due to unbalanced exciton recombination. The EL spectra of the devices were slightly broader than that of the previous report47, this could be attributed to the self-accumulation of the dopant because of differences in concentration, and self-absorption of other layers in the devices, such as BPBPA, on the basis of the narrowed spectra of the PtON1-doped thin films (Supplementary Fig. 56).
To solve the shortcoming and further improve the device performance, co-host systems of BO1b together with bipolar host 26mCPy (device 5), p-tpye hosts mCP (device 6) or mCBP (device 7) were employed to improve the exciton balance. As expected, devices 5, 6, and 7 demonstrated enhanced color purities, improved Lmaxs, and also significantly increased EQEs with dramatically reduced efficiency roll-offs compared to their corresponding single host devices 1–4 (Fig. 7g–i, Supplementary Fig. 57, Table 2). In particular, BO1b:mCBP-based device 7 achieved a peak EQE of 27.1%, and retained high EQEs of 25.2%, 21.8%, and 15.6% at 100, 1000 and 5000 cd/m2, respectively, which was significantly better than the previous report with the same emitter47 (Table 2). Surprisingly, BO1b:mCBP-based device 7 also achieved an Lmax value of up to 15722 cd/m2, which was a 1.34-fold enhancement compared to mCBP-based device 3 and, and about 2-fold for BO1b-based device 4 in the same device settings (Table 2). This was attributed the improved charge balance in the EML and the reduction of charge accumulation at the interface between the EBL/EML or EML/HBL.
Brightness is also an important parameter of device performance, and high brightness is an urgent demand for the outdoor applications of display in smartphone. Our previously reported blue OLED employing PtON7-dtb as emitter demonstrated a high color purity and a high peak EQE, but suffered from the issue of large efficiency roll-off and small Lmax (~1555 cd/m2)48. Narrow-band blue emitter PtON7-dtb48-based device 8 with co-host of BO1b:mCBP was also fabricated (Fig. 7j–l, Table 2). Device 8 exhibited a narrow EL spectrum peaking at 453 nm with a FWHM of 28 nm and a CIEy of 0.088, and also realized a peak EQE of 27.6% with small efficiency roll-off, and retained high EQEs of 20.0%, 16.0% and 7.9% at 100, 1000 and 5000 cd/m2, respectively; device 8 also achieved an Lmax of 5670 cd/m2. Device 8 showed larger efficiency roll-off than that of device 7 with the same device structure due to the slightly higher ET1 and longer τ of PtON7-dtb compared to PtON155. PtON-TBBI-based deep-blue OLED demonstrated exceptionally long operational lifetime, but suffered from low color purity (CIEy = 0.197) and small Lmax (~2000 cd/m2)46. Employing PtON-TBBI (ET1 = 2.78 eV, Supplementary Fig. 58) with short τ of 2.01 µs as emitter46, device 9 demonstrated a peak EQE of 28.0%, which also represented the record-high EQE among Pt(II)-based deep-blue OLEDs with CIEy < 0.2046,47,48,49,56,57,58,59,60,61,62,63,64, and was also among the highest EQE in the reported Ir(III)-based deep blue OLEDs65,66,67,68,69,70 (Table 2, Supplementary Figs. 59, 60, Supplementary Tables 7, 8). Device 9 also showed narrow EL spectrum with a small FWHM of 21 nm, resulting in a huge color purity (CIEy = 0.104); moreover, a Lmax of 9377 cd/m2 was also achieved (Table 2).
Then, other dBO-PAHs with high ET as hosts in deep-blue OLEDs were also investigated (Fig. 8, Supplementary Fig. 55, Table 2). The LUMO and HOMO levels of the dBO-PAHs were first calculated through their reduction potentials and photophysical properties (Supplementary Fig. 61, Supplementary Table 6). BO1c (ET1 = 3.02 eV)-based device 10 and BO1g (ET1 = 3.01 eV)-based device 11 using PtON1 as emitter also demonstrated good device performances with peak EQEs of 27.8% and 25.1%, retained high EQEs of 20.0% and 19.2% at 1000 cd/m2, respectively; and they also achieved high Lmaxs of 27219 and 22683 cd/m2, respectively; the Lmaxs were greatly increased compared to device 7 (15722 cd/m2). Importantly, the Lmax (27219 cd/m2) of device 10 represented the record-high Lmax among Pt(II), Ir(III) and BN-PAHs-based deep-blue OLEDs with CIEy < 0.20 (Fig. 7b, Supplementary Figs. 59, 60, 62, 63, Supplementary Tables 7–9)46,47,48,49,56,57,58,59,60,61,62,63,64. By comparison, PtON1:BO2 device 12 and PtON1:BO3a device 13 exhibited significantly low EQEs and Lmaxs; this was attributed to the similar ET1 of BO2 (2.88 eV) and BO3a (2.88 eV) compared to PtON1 (2.82 eV48), which could not enable efficient energy transfer from host to emitter molecules. However, BO2 and BO3a, as well as BO6 (ET1 = 2.81 eV) could be acted as hosts for PtON-TBBI (ET1 = 2.78 eV)-based deep-blue OLEDs, devices 14, 15 and 16 showed narrow EL spectra with FWHM values of 21–22 nm, they also realized peak EQEs of 22.2%, 19.6% and 21.7%, and high Lmaxs of 14481, 15765 and 10071 cd/m2, respectively. Last, the operational lifetimes of some deep-blue OLEDs were measured (Supplementary Fig. 64); because of the poor stability of DPEPO, they showed LT50 with L0 = 500 cd/m2 of 0.2–1.0 h. However, PtON1:BO1c:mCBP device 10 and PtON1:BO1g:mCBP device 11 exhibited obviously longer operational lifetimes than those of PtON1:26mCPy device 1, this revealed that the co-hosts-based OLEDs with improved charge balance increased the operational lifetime. Moreover, although PtON1 (ET1 = 2.82 eV)48 had higher ET1 than that of PtON-TBBI (ET1 = 2.78 eV), PtON1-based devices 10 and 11 also demonstrated significantly longer operational lifetimes compared to PtON-TBBI-based devices 14, 15 and 16, which revealing that the CIEy value also had a great effect on the operational lifetime. The device performance should be further improved by optimizing the ratio of n-type dBO-PAHs and p-type host in the EML and employing advanced functional materials of other layers. The above studies demonstrate that BO1b and other dBO-PAHs with high ET1, e.g., BO1a, BO1c, BO1e, BO1g, BO2, BO3a, BO3b and BO6 (Table 1), can act as n-tpye host materials for high-brightness deep-blue OLEDs. This study provides a design concept for the development of host materials for deep-blue OLEDs, and also broadens the application of the dBO-PAHs in the field of organic electronics.
Discussion
In this work, we developed a facile synthesis of dBO-PAHs through an efficient one-pot strategy consisting of tandem demethylation-electrophilic borylation-nucleophilic substitution reactions. Using this method, six types of dBO-PAHs were successfully synthesized with high regioselectivity and efficient skeletal editing. The dBO-PAHs showed high chemical and thermal stabilities. Single crystal X-ray diffraction analyses suggested that the enforced planarity of the BO cores and Mes groups effectively enhanced their chemical stability, and also greatly affected their solid-state stacking. Theoretical calculations revealed that the BO-fused rings showed low aromaticity and could effectively regulate the electronic properties of the dBO-PAHs. The dBO-PAHs exhibited strong fluorescence emission at room temperature in dichloromethane and achieved a ΦPL and kr values of up to 95% and 6.8 × 108 s−1, respectively. In contrast, at 77 K in 2-MeTHF, most dBO-PAHs exhibited single-component, dual-emissions with nanosecond fluorescence (1.2–12.6 ns) and second-level UOP (0.3–5.0 s); importantly, the long τp enabled the dBO-PAHs to exhibit low-temperature ultralong afterglow of up to 20 s after UV light turn-off. Moreover, the BO incorporations endowed the dBO-PAHs to possess enlarged Eg and an increased ET1, making them ideal n-type host materials for high-brightness and high-efficiency deep-blue OLEDs; compared to single host device, deep-blue OLEDs employing BO1b:mCBP as co-hosts demonstrated significantly enhanced Lmaxs, EQEs and reduced efficiency roll-offs; PtON1-based device 10 achieved the record-high Lmax (27219 cd/m2) and PtON-TBBI-based device 9 realized the record-high EQE (28.0%) among Pt(II)-based deep-blue OLEDs with CIEy < 0.20. These results demonstrate the great potential of the dBO-PAHs as low-temperature ultralong afterglow and n-type host materials in optoelectronic applications, and can also help to understand the electronic and optical properties of the dBO-PAHs.
Methods
Synthesis and structure characterization
Unless noted, all commercial reagents were purchased and used as received without further purification. Toluene and n-hexane were dried with CaH2 reflux, and distillation before use. 1 H NMR spectra were recorded at 400 or 500 MHz Bruker “AVANCE III” nuclear magnetic resonance system in CDCl3 or DMSO-d6 solutions and chemical shifts were referenced to tetramethylsilane (TMS) or residual protiated solvent. If CDCl3 was used as solvent, 1H and 13C NMR spectra were recorded with TMS (δ = 0.00 ppm) and CDCl3 (δ = 77.00 ppm) as internal references, respectively. If DMSO-d6 was used as solvent, 1H and 13C NMR spectra were recorded with TMS (δ = 0.00 ppm) and DMSO-d6 (δ = 39.52 ppm) as internal references, respectively. All of the new compounds were analyzed for HRMS on a Waters mass spectrometer using electrospray ionization in the positive ion mode of ESI-Q-TOF.
General experimental procedure for the synthesis of dBO-PAHs
The dimethoxy compound was added to a dry three-necked flask equipped with a magnetic stir bar and a condensor. The flask was then evacuated and backfilled with nitrogen, this evacuation and backfill procedure was repeated twice. Then solvent toluene and n-hexane were added under a nitrogen atmosphere, then BBr3 was added dropwise. The mixture was stirred at room temperature for 18‒40 h monitoring by TLC until the demethylation was completely. Then AlCl3 was added quickly (The AlCl3 is highly hygroscopic and should be weighed quickly!), the flask was placed in oil bath (75 oC) and stirred for 8‒10 h, and then the mixture was cooled down to room temperature. Mesitylmagnesium bromide (MesMgBr) was then added dropwise, and the mixture was then stirred at room temperature for another 1‒10 hours monitoring by TLC until the reaction was completely. The reaction mixture was concentrated under reduced pressure, and the residue was purified through column chromatography on silica gel using petroleum ether/dichloromethane as eluent to afford the desired dBO-PAH compound. Please see the Supporting Information for the synthetic details of the dBO-PAHs.
X-ray crystallography
X-ray diffraction data were collected at 170 K on a Bruker D8 Venture diffractometer using graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å) from a rotating anode generator. The crystal structures were solved by ShelXT or ShelXS and refined with full-matrix least-squares methods with anisotropic thermal parameters for all non-hydrogen atoms on F2 using SHELXL-2015. Hydrogen atoms were found from different Fourier maps but placed in calculated positions and were refined isotropically using a riding model71,72,73. The crystal data as well as the details of data collection and refinement are summarized in Supplementary Tables 1–4.
Thermal properties
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were performed by TA-Q50. The TGA curve was measured at a heating rate of 10 °C/min from RT to 500 °C under nitrogen flow, after eliminating residual thermal history of compound.
Quantum chemical calculations
The theoretical calculations were performed using Gaussian 09. The molecular geometries of ground states (S0) were optimized with the density functional theory (DFT) method. The DFT calculations were performed using a B3LYP function with a basis set of 6-31 G(d) for C, H, O, and B atoms. The spin-orbit coupling (SOC) matrix elements between S1 and Tn states of BO1a, BO1d, and BO6 were evaluated using PySOC at the B3LYP/6-31 G(d,p) level74.
Electrochemistry
Cyclic voltammetry and different pulsed voltammetry were performed using a CH1760E electrochemical analyzeraccording previous report. 0.1 M tetra-n-butylammonium hexafluorophosphate was used as the supporting electrolyte, anhydrous N,N-dimethylformamide, was used as the solvents for the Eox and Ered measurements, and the solutions were bubbled with nitrogen for 15 min prior to the test. Silver wire, platinum wire and glassy carbon were used as pseudoreference electrode, counter electrode, and working electrode, respectively. The scan rate was 300 mV/s. The redox potentials are based on the values measured from different pulsed voltammetry and are reported relative to an internal reference ferrocenium/ferrocene (Cp2Fe/Cp2Fe+). The reversibility of reduction or oxidation was determined using CV.
Photophysical measurements
The absorption spectra were measured on an Agilent 8453 UV–VS Spectrometer. Steady-state emission experiments and lifetime measurements were performed on a Horiba Jobin Yvon FluoroLog-3 spectrometer. Low temperature (77 K) emission spectra and lifetimes were measured in 2-MeTHF cooled with liquid nitrogen.
OLED fabrication and characterization
All devices were fabricated by vacuum thermal evaporation, and were tested outside glove box after encapsulation. Prior to deposition, the prepatterned ITO-coated glass substrates were cleaned by subsequent sonication in deionized water, acetone, and isopropanol. The metal layer and organic layers were fabricated by vacuum thermal evaporation on the cleaned indium-tin-oxide (ITO) glass substrate under vacuum (<4 × 10–4 Pa) with 4 Å/s deposition rate for aluminum cathode and 2 Å/s for organic layers. The device areas were 9 mm2 (3 mm × 3 mm). The current density–voltage–luminance characteristics of OLEDs were measured using a Keithey 2400 Source meter and a Keithey 2000 Source multimeter equipped with a calibrated silicon photodiode. The electroluminescence (EL) spectra were recorded with a multichannel spectrometer (PMA12, Hamamatsu Photonics).
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its Supplementary Information files. Extra data are available from the corresponding author upon request. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1954663 (BO1a), 1954662 (BO2), 1954665 (BO3a), 2290997 (BO3b-OH), 1954664 (BO3d), 1954661 (BO4b), 1966566 (BO4d), 1968760 (BO4e), 2280610 (BO5) and 2029823 (BO6). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. The new crystallographic structures of target molecules are also available within the accompanied files. Source data are provided with this paper [data https://doi.org/10.6084/m9.figshare.24146901]75 Source data are provided with this paper.
References
Borissov, A. et al. Recent advances in heterocyclic nanographenes and other polycyclic heteroaromatic compounds. Chem. Rev. 122, 565–788 (2022).
Navalon, S. et al. Carbocatalysis by graphene-based materials. Chem. Rev. 114, 6179–6212 (2014).
Miao, J., Wang, Y., Liu, J. & Wang, L. Organoboron molecules and polymers for organic solar cell applications. Chem. Soc. Rev. 51, 153–187 (2022).
Bendikov, M., Wudl, F. & Perepichka, D. F. Tetrathiafulvalenes, oligoacenenes, and their buckminsterfullerene derivatives: the brick and mortar of organic electronics. Chem. Rev. 104, 4891–4946 (2004).
Min, Y., Dou, C., Liu, D., Dong, H. & Liu, J. Quadruply B←N-fused dibenzo-azaacene with high electron affinity and high electron mobility. J. Am. Chem. Soc 141, 17015–17021 (2019). 2019.
Li, W. et al. BN-Anthracene for high-mobility organic optoelectronic materials through periphery engineering. Angew. Chem. Int. Ed 61, e202201464 (2022).
Kothavale, S. S. & Lee, J. Y. Three- and four-coordinate, boron-based, thermally activated delayed fluorescent emitters. Adv. Opt. Mater. 8, 2000922 (2020).
Ha, J. M., Hur, S. H., Pathak, A., Jeong, J.-E. & Woo, H. Y. Recent advances in organic luminescent materials with narrowband emission. NPG Asia Mater. 13, 53 (2021).
Kondo, Y. et al. Narrowband deep-blue organic light-emitting diode featuring an organoboron-based emitter. Nat. Photonics 13, 678–682 (2019).
Suresh, S. M. et al. A deep blue B,N-doped heptacene emitter that shows both thermally activated delayed fluorescence and delayed fluorescence by triplet–triplet annihilation. J. Am. Chem. Soc. 142, 6588–6599 (2020).
Hu, Y. X. et al. Efficient selenium-integrated TADF OLEDs with reduced roll-off. Nat. Photonics 16, 803–810 (2022).
Farrell, J. M. C. et al. Tunable low-LUMO boron-doped polycyclic aromatic hydrocarbons by general one-pot C–H borylations. J. Am. Chem. Soc. 141, 9096–9104 (2019).
Ito, M., Sakai, M., Ando, N. & Yamaguchi, S. Electron-deficient heteroacenes that contain two boron atoms: near-infrared fluorescence based on a push–pull effect. Angew. Chem. Int. Ed. 60, 21853–21859 (2021).
Giustra, Z. X. & Liu, S.-Y. The state of the art in azaborine chemistry: new synthetic methods and applications. J. Am. Chem. Soc. 140, 1184–1194 (2018).
Ju, C.-W. et al. Modular synthesis of pentagonal and hexagonal ring-fused NBN-phenalenes leading to an excited-state aromatization-induced structural planarization molecular library. J. Am. Chem. Soc. 143, 5903–5916 (2021).
Hatakeyama, T., Hashimoto, S., Seki, S. & Nakamura, M. Synthesis of BN-fused polycyclic aromatics via tandem intramolecular electrophilic arene borylation. J. Am. Chem. Soc. 133, 18614–18617 (2011).
Nakatsuka, S., Yasuda, N. & Hatakeyama, T. Rh(III)-catalyzed redox-neutral unsymmetrical C–H alkylation and amidation reactions of N-phenoxyacetamides. J. Am. Chem. Soc. 2018 140, 13562–13565 (2018).
Dewar, M. J. S. & Dietz, R. New heteroaromatic compounds. Part V. Some 9,10-boroxarophenanthrenes. J. Chem. Soc. 1344–1347 (1960).
Budanow, A. et al. 10,9-Oxaboraphenanthrenes as luminescent fluorophores. Tetrahedron 72, 1477–1484 (2016).
Numano, M. et al. Synthesis of boronate-based benzo[fg]tetracene and benzo[hi]hexacene via demethylative direct borylation. Chem. Eur. J 22, 11574–11577 (2016).
Katayama, T. et al. Two-step synthesis of boron-dused double helicenes. J. Am. Chem. Soc. 138, 5210–5213 (2016).
Wang, X.-Y., Narita, A., Zhang, W., Feng, X. & Müllen, K. Synthesis of stable nanographenes with OBO-doped zigzag edges based on tandem demethylation-electrophilic borylation. J. Am. Chem. Soc. 138, 9021–9024 (2016).
Kaehler, T., Bolte, M., Lerner, H.-W. & Wagner, M. Introducing perylene as a new member to the azaborine family. Angew. Chem. Int. Ed. 58, 11379–11384 (2019).
Li, G., Lou, W., Wang, D., Deng, C. & Zhang, Q. Difluoroboron-enabled thermally activated delayed fluorescence. ACS Appl. Mater. Interfaces 11, 32209–32217 (2019).
Hitchcock, P. B., Jasim, H. A., Lappert, M. F. & Williams, H. D. Bulky metal aryloxides, arylamides and sulphur and phosphorus analogues—III. Aluminium compounds derived from 2,4,6-TRI-t-butylaniline; X-ray structure of [AIMe{μ-NHC6H2But2-4,6-C(Me)2CH2-2}]2. Polyhedron 9, 245–251 (1990).
Blount, J. F., Finocchiaro, P., Gust, D. & Moslow, K. Conformational analysis of triarylboranes. J. Am. Chem. Soc. 95, 7019–7029 (1973).
Brown, H. C. & Racherla, U. S. Organoboranes. 44. A convenient, highly efficient synthesis of triorganylboranes via a modified organometallic route. J. Org. Chem. 51, 427–432 (1986).
Fratini, S., Nikolka, M., Salleo, A., Schweicher, G. & Sirringhaus, H. Charge transport in high-mobility conjugated polymers and molecular semiconductors. Nat. Mater. 19, 491–502 (2020).
Turro, N. J., Ramamurthy, V., Scaiano & J. C. Modern Molecular Photochemistry of Organic Molecules University 195−200 (Science Books, 2009).
She, Y. et al. Tetradentate platinum(II) and palladium(II) complexes containing fused 6/6/6 or 6/6/5 metallocycles with azacarbazolylcarbazole-based ligands. Inorg. Chem. 60, 12972–12983 (2021).
Sanchez-Barragan, I. et al. Room-temperature phosphorescence (RTP) for optical sensing. Trends Anal. Chem. 25, 958–567 (2006).
Zhang, G., Palmer, G. M., Dewhirst, M. W. & Fraser, G. L. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat. Mater. 8, 747–751 (2009).
Zhou, Y. et al. Long-lived room-temperature phosphorescence for visual and quantitative detection of oxygen. Angew. Chem. Int. Ed. 58, 12102–12106 (2019).
Lojpur, V., Nikolić, G. & Dramićanin, M. D. Luminescence thermometry below room temperature via up-conversion emission of Y2O3:Yb3+, Er3+ nanophosphors. J. Appl. Phys. 115, 203106 (2014).
Kreder, M. J., Alvarenga, J., Kim, P. & Aizenberg, J. Design of anti-icing surfaces: smooth, textured or slippery? Nat. Rev. Mater. 1, 15003 (2016).
Yang, X. et al. Low-temperature red long-persistent luminescence of Pr3+ doped NaNbO3 with a perovskite structure. Lumin. 208, 290–295 (2019).
Du, J. & Poelman, D. Identifying Near-infrared persistent luminescence in Cr3+-doped magnesium gallogermanates featuring afterglow emission at extremely low temperature. Adv. Opt. Mater. 8, 1901848 (2020).
Zhao, W., He, Z. & Tang, B. Z. Room-temperature phosphorescence from organic aggregates. Nat. Rev. Mater. 5, 869–885 (2020).
Yang, J. et al. AIEgen with fluorescence–phosphorescence dual mechanoluminescence at room temperature. Angew. Chem. Int. Ed. 56, 880–884 (2017).
Gu, L. et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photonics 13, 406–411 (2019).
Zhu, H. I. et al. Cyclization-promoted ultralong low-temperature phosphorescence via boosting intersystem crossing. J. Am. Chem. Soc. 143, 2164–2169 (2021).
Zhang, X. et al. Ultralong phosphorescence cellulose with excellent anti-bacterial, water-resistant and ease-to-process performance. Nat. Commun. 13, 1117 (2022).
Li, G. et al. Efficient deep-blue organic light-emitting diodes employing difluoroboron-enabled thermally activated delayed fluorescence emitters. J. Mater. Chem. C 8, 17464–17473 (2020).
Li, G. et al. Difluoroboron-enabled thermally activated delayed fluorescence. ACS Appl. Mater. Interfaces 11, 32209–32217 (2019).
Li, P. et al. Four-coordinate boron emitters with tridentate chelating ligand for efficient and stable thermally activated delayed fluorescence organic light-emitting devices. Angew. Chem. Int. Ed. 58, 9088–9094 (2019).
Sun, J. et al. Exceptionally stable blue phosphorescent organic light-emitting diodes. Nat. Photonics 16, 212–218 (2022).
Hang, X. C., Fleetham, T., Turner, E., Brooks, J. & Li, J. Highly efficient blue-emitting cyclometalated platinum(II) complexes by judicious molecular design. Angew. Chem. Int. Ed. 52, 6753–6756 (2013).
Fleetham, T., Li, G., Wen, L. & Li, J. Efficient “pure” blue OLEDs employing tetradentate Pt complexes with a narrow spectral bandwidth. Adv. Mater. 26, 7116–7121 (2014).
Li, G., Fleetham, T., Turner, E., Hang, X.-C. & Li, J. Highly efficient and stable narrow-band phosphorescent emitters for OLED applications. Adv. Opt. Mater. 3, 390–397 (2015).
Li, G. et al. Novel carbazole/fluorene-based host material for stable and efficient phosphorescent OLEDs. ACS Appl. Mater. Interfaces 11, 40320–40331 (2019).
Williams, E. L., Haavisto, K., Li, J. & Jabbour, G. E. Excimer-based white phosphorescent organic light-emitting diodes with nearly 100% internal quantum efficiency. Adv. Mater. 19, 197–202 (2007).
Klimes, K., Zhu, Z.-Q. & Li, J. Efficient blue phosphorescent OLEDs with improved stability and color purity through judicious triplet exciton management. Adv. Funct. Mater. 29, 1903068 (2019).
Li, G. et al. Tetradentate platinum(II) complexes for highly efficient phosphorescent emitters and sky blue OLEDs. Chem. Mater. 32, 537–548 (2020).
Jeon, S. O., Jang, S. E., Son, H. S. & Lee, H. Y. External quantum efficiency above 20% in deep blue phosphorescent organic light-emitting diodes. Adv. Mater. 23, 1436–1441 (2011).
Li, G., Wolfe, A., Brooks, J., Zhu, Z.-Q. & Li, J. Modifying emission spectral bandwidth of phosphorescent platinum(II) complexes through synthetic control. Inorg. Chem. 56, 8244–8256 (2017).
Li, K. et al. Blue electrophosphorescent organoplatinum(II) complexes with dianionic tetradentate bis(carbene) ligands. Chem. Commun. 47, 9075–9077 (2011).
Fleetham, T., Wang, Z. & Li, J. Efficient deep blue electrophosphorescent devices based on platinum(II) bis(n-methyl-imidazolyl)benzene chloride. Org. Electron. 13, 1430–1435 (2012).
Wang, X. et al. Highly efficient deep-blue electrophosphorescent Pt(II) compounds with non-distorted flat geometry: tetradentate versus macrocyclic chelate ligands. Adv. Funct. Mater. 27, 1604318 (2017).
Ma, H. et al. High-color-purity and efficient solution-processable blue phosphorescent light-emitting diodes with Pt(II) complexes featuring 3ππ* transitions. Mater. Chem. Front. 3, 2448–2454 (2019).
Zhu, L. et al. Tetradentate Pt(II) complexes for spectrum-stable deep-blue and white electroluminescence. Adv. Optical. Mater. 8, 2000406 (2020).
Chen, Y. et al. Ultrapure blue phosphorescent organic light-emitting diodes employing a twisted Pt(II) complex. ACS Appl. Mater. Interfaces. 13, 52833–52839 (2021).
Huh, J.-S., Sung, M.-J., Kwon, S.-K., Kim, Y.-H. & Kim, J.-J. Highly efficient deep blue phosphorescent OLEDs based on tetradentate Pt(II) complexes containing adamantyl spacer groups. Adv. Funct. Mater. 31, 2100967 (2021).
Park, H. J., Jang, J.-H., Lee, J.-H. & Hwang, D.-H. Highly efficient deep-blue phosphorescent OLEDs based on a trimethylsilyl-substituted tetradentate Pt(II) complex. ACS Appl. Mater. Interfaces. 14, 34901–34908 (2022).
Huh, J.-S. et al. Control of the horizontal dipole ratio and emission color of deep blue tetradentate Pt(II) complexes using aliphatic spacer groups. Chem. Eng. J. 450, 137836 (2022).
Lu, K.-Y. et al. Wide-range color tuning of iridium biscarbene complexes from blue to red by different N^N ligands: an alternative route for adjusting the emission colors. Adv. Mater. 23, 4933–4937 (2011).
Lee, J. et al. Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency. Nat. Mater. 15, 92–98 (2016).
Park, H. Y. et al. External quantum efficiency exceeding 24% with CIEy value of 0.08 using a novel carbene-based iridium complex in deep-blue phosphorescent organic light-emitting diodes. Adv. Mater. 32, 2002120 (2020).
Shin, H. et al. Controlling horizontal dipole orientation and emission spectrum of Ir complexes by chemical design of ancillary ligands for efficient deep-blue organic light-emitting diodes. Adv. Mater. 31, 1808102 (2019).
Qin, Y. et al. Facially Coordinated, Tris-bidentate purin-8-ylidene Ir(III) complexes for blue electrophosphorescence and hyperluminescence. Adv. Opt. Mater. 10, 2201633 (2022).
Kumaresan, R. et al. High performance solution-processed deep-blue phosphorescence organic light-emitting diodes with EQE over 24% by employing new carbenic Ir(III) complexes. Adv. Opt. Mater. 10, 2101686 (2022).
Sheldrick, G. M. A short history of ShelX. Acta Crystallogr. A64, 339–341 (2008).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Sheldrick, G. M. ShelXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Gao, X. et al. Evaluation of spin-orbit couplings with linear-response time-dependent density functional methods. J. Chem. Theory Comput. 13, 515–524 (2017).
Li, G. et al. Double boron–oxygen-fused polycyclic aromatic hydrocarbons: skeletal editing and applications as organic optoelectronic materials. figshare. Dataset. https://doi.org/10.6084/m9.figshare.24146901 (2023).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 22178319, G.L.; 21878276, G.L. and 22138011, Y.-B.S.). We thank Prof. Qisheng Zhang and Dr. Shaohai Chen’s helpful discussion. We also thank Jiyong Liu from Zhejiang University for the help of the single crystal measurements and analyses and Zhejiang Hongwu Technology Co., Ltd for the device fabrications and measurements.
Author information
Authors and Affiliations
Contributions
G.L. and Y.-B.S. initiated and supervised the project. G.L. designed the materials and analyzed the data. K.X., J.Z., Q. Chu, J.D. Q. Chen, and Y.Y. synthesized and characterized the doubly BO-embedded emitters and hosts. K.X., J.Z., J.D., Q. Chen, and Y.Y. performed the photophysical and electrochemical measurements. X.F., K.X., and Y.-F.Y. performed the computational calculation. W.L. fabricated the OLEDs and measured the device performance. G.L. and K.X. contributed to the manuscript writing. All authors discussed the progress of the research and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, G., Xu, K., Zheng, J. et al. Double boron–oxygen-fused polycyclic aromatic hydrocarbons: skeletal editing and applications as organic optoelectronic materials. Nat Commun 14, 7089 (2023). https://doi.org/10.1038/s41467-023-42973-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-42973-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.