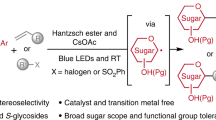
Abstract
Photochemical glycosylation has attracted considerable attention in carbohydrate chemistry. However, to the best of our knowledge, visible-light-promoted glycosylation via photoactive glycosyl donor has not been reported. In the study, we report a photosensitizer-free visible-light-mediated glycosylation approach using a photoactive 2-glycosyloxy tropone as the donor. This glycosylation reaction proceeds at ambient temperature to give a wide range of O-glycosides or oligosaccharides with yields up to 99%. This method is further applied in the stereoselective preparation of various functional glycosyl phosphates/phosphosaccharides, the construction of N-glycosides/nucleosides, and the late-stage glycosylation of natural products or pharmaceuticals on gram scales, and the iterative synthesis of hexasaccharide. The protocol features uncomplicated conditions, operational simplicity, wide substrate scope (58 examples), excellent compatibility with functional groups, scalability of products (7 examples), and high yields. It provides an efficient glycosylation method for accessing O/N-glycosides and glycans.
Similar content being viewed by others
Introduction
Carbohydrates are widely distributed in nature and play pivotal roles in bacterial and viral infection, intercellular recognition, immune regulation, inflammation development, cancer cell metabolism, and other physiological or pathological processes1,2. Carbohydrate-based drugs and vaccines have been extensively studied to treat and prevent various diseases3. Thus, carbohydrate synthesis has attracted considerable attention owing to the numerous biological and pharmacological activities of carbohydrates. Glycosylation, the attachment of a glycosyl donor to a glycosyl acceptor via a glycosidic bond, is a central focus for synthesizing carbohydrates and is characterized by challenging reactions. The glycosyl donor bearing a leaving group at the anomeric position significantly influences the outcome of glycosylation reactions4,5,6. In the past decades, a series of effective glycosyl donors, such as glycosyl halides7, glycosyl imidates8, thioglycosides9, glycosyl phosphites/phosphates10, glycals11, benzoyl/phenyl glycosides12,13, and others14,15,16,17, have been used in the chemical synthesis of glycans18,19,20,21. However, at present, no general glycosylation method for glycan synthesis has been reported. Novel and efficient glycosyl donors are highly demanded in constructing complex glycans.
Visible-light-mediated reactions have proven to be reliable and efficient for organic synthesis22,23,24,25,26. Photo-driven glycosylation has attracted significant attention in organic and carbohydrate chemistry27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50. Currently, visible-light-induced glycosylation reactions are achieved by either photoactivating a photosensitizer or using a stoichiometric activator first to generate an intermediate, which facilitates the departure of the leaving group, forming the glycosyl cation (Fig. 1A). Compared with traditional chemical glycosylation reactions, photo-glycosylation has historically been underexplored27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50. Thus, we envisaged that a direct light-activation of a photoactive-leaving group might greatly improve the photochemical glycosylation efficiency. However, to the best of our knowledge, visible-light-promoted glycosylation via a photoactive glycosyl donor has not been reported. Thus, we directed our efforts toward designing a photoactive-leaving group to fill this gap.
The capacity of the chromophoric leaving group to absorb light at the irradiation wavelength (preferably visible light) is one of the fundamental prerequisites for a photoactive glycosyl donor. Tropolone and its derivatives are a class of non-benzenoid aromatic compounds known for their diverse biological activities51. Tropolone itself exhibits fluorophoric properties52,53. Extensive research has been conducted on the photocyclization and photocycloaddition of tropolone (Fig. 1B)54,55. Tropolonate salts are suitable for acyl-transfer catalysts under visible-light conditions56. However, tropolone has rarely been employed in carbohydrate chemistry. We envisaged that tropolone might be an ideal photoactive-leaving group for visible-light-induced glycosylation.
In this work, we report an efficient visible-light- promoted glycosylation using 2-glycosyloxy tropones as the donors.
Results
Reaction Optimization
We commenced our investigation by examining the glycosylation reaction of glycosyl donor 1a with glycosyl acceptor 2a (Table 1 and Supplementary Tables 1–4). When a mixture of 1a (0.075 mmol) and 2a (0.05 mmol) in 1,2-dichloroethane was stirred in the absence of light for 3 h, no desired product 3a was detected (Table 1, entry 1). To our delight, we indeed isolated the desired product 3a in 22% isolated yield when the reaction was exposed to light with a wavelength of 310–320 nm (Table 1, entry 2). We systematically investigated the impact of various irradiation wavelengths, including 365–370 nm, 380–390 nm, 415–420 nm, 430–435 nm, and 450–465 nm (Table 1, entries 3–7). We found light irradiation was essential for this reaction. Visible light was superior to ultraviolet light. Finally, blue LEDs (15 W) were chosen as this reaction’s light source, and the reaction yield was 67% (Table 1, entry 7). A decrease in illumination intensity led to a reduction in the reaction yield (Table 1, entries 8–10). In addition, a modest excess of glycosyl donor 1a was necessary to achieve a satisfactory isolated yield (82%, Table 1, entry 11). A catalytic NaOTf could shorten the reaction time and improve the reaction yield (91%, Table 1, entry 12). The role of NaOTf might provide an anion and generate a highly reactive glycosyl triflate57.
In the following experiments, a more challenging reaction between donor 1b and acceptor 2b was examined (Table 1, entries 13–25; Supplementary Table 5). A yield of 71% for the formation of disaccharide 3b suggests that there is room for improvement in the reaction conditions (Table 1, entry 13). As a result, we checked other salt triflates and sodium salts with different anions, such as NaB(3,5-(CF3)2Ph)4, NaSO2CF3, NaBF4, NaNTf2, TMSOTf, Bu4NOTf, KOTf, and NaSO4Me (Table 1, entries 14–21; Supplementary Table 5). The catalytic performance of sodium triflate and trimethylsilyl triflate was superior to other additives (Table 1, entries 13 and 21). Although the control experiment showed that the reaction could still be promoted by TMSOTf in the dark for 12 h, it is evident that the overall reaction efficiency remained low (44%, Table 1, entries 22–25). This indicates that light plays an important role in the reaction. Considering the glycosylation efficiency on the disarmed donor, we would choose TMSOTf as the additive with the loading of 10 mol% (Table 1, entry 21). Solvents other than dichloromethane reduced the yield of the desired product 3a (Supplementary Table 3). Moreover, the molecular sieves suppressed this glycosylation reaction (Table S4, entry 10; Supplementary Table 5, entries 23–24).
Evaluation of acceptor scope for photo-O-glycosylation
Alongside the optimized reaction conditions, we investigated the acceptor scope of the visible-light-promoted O-glycosylation reaction (Fig. 2). First, a perbenzoylated 2-galactosyloxy tropone (disarmed donor)58 was chosen to examine the scope of the acceptors. As summarized in Fig. 2A, primary, secondary, and tertiary alcohols were suitable glycosyl acceptors under the standard reaction conditions, resulting in excellent yields of the desired glycosides 3c–3g. The reaction of perbenzoylated 2-galactosyloxy tropone with various glycosyl acceptors bearing a free hydroxyl group at C-6, C-2, C-3, and C-4 positions generated the disaccharides 3h–3l with yields ranging from 88% to 96%. The glycosyl acceptor with a p-methylphenylthio-leaving group for further glycosylation reaction was also an effective reactant in this protocol9, generating the corresponding product 3m with 98% yield. Low-reactive acceptors, such as phenols and carboxylic acids, were all amenable substrates in the visible-light-promoted glycosylation reaction. 4-Methoxy phenol, 4-hydroxycoumarin, and 6-methoxy-2-naphthol underwent smooth coupling with the disarmed donor to generate the phenolic glycosides 3n–3p in 92% to 96% yields. No C-glycoside byproducts arising from O → C-glycoside rearrangement were detected in the crude mixtures59. In addition, both aromatic acid and alkyl acid were effectively reacted under the standard reaction conditions, generating 3q and 3r with excellent yields.
Evaluation of donor scope for photo-O-glycosylation
Second, the scope of the donors was explored for O-glycosylation (Fig. 2B). The efficiency of the transformation was well illustrated by the reaction of the disarmed glycosyl donors with the low-reactive glycosyl acceptors58, especially the glycosyl acceptors bearing a free hydroxyl group at C-4 positions. A series of perbenzoylated 2-glycosyloxy tropone donors, including glucosyl, mannosyl, arabinosyl, xylosyl, rhamnosyl, ribosyl, and amino-glucosyl donors, successfully underwent visible-light-promoted O-glycosylation, generating the corresponding disaccharides (4a–4i) with excellent yields. The peracetylated galactosyl donor and glucosyl donor were appropriate substrates for glycosylation, albeit in moderate to good yields of the products (4j–4l, 62–76%). Some acetylated acceptors were isolated as the byproducts, with about 10% yields arising from an acetyl transfer from the donors. This type of byproduct was formed with a yield up to 42% when a thioglycoside donor was used60. The glycosylation of perbenzylated 2-galactosyloxy tropone donor generated disaccharides 4m with a mixture of α/β anomers with good yield. Participating solvents could facilitate the preferential formation of one anomer6, that is, acetonitrile favors the β-anomer, while diethyl ether favors the α-anomer. Tert-Butyldiphenylsilyl (4n) was tolerated well under the reaction conditions. Notably, perbenzoylated 2-lactosyloxy tropone was a viable donor, yielding 78% of the desired trisaccharide 4o.
Synthesis of glycosyl phosphosaccharides
Given the above results, we examined the impact of visible-light-promoted O-glycosylation on the preparation of glycosyl phosphosaccharides (Fig. 3A). Phosphoglycans represent an important class of glycopolymers on the outer membrane of bacteria, yeasts, and protozoa containing anomeric phosphodiester linkages61. The synthesis of glycosyl phosphosaccharides is intrinsically challenging because the glycosylation of a glycosyl donor with a phosphoric acid usually forms a pair of diastereoisomers, especially the construction of α-glycosyl phosphosaccharides62,63. We first explored the reactivity of perbenzoylated 2-glycosyloxy tropone donor with phosphate nucleophiles. A series of β-glycosyl phosphosaccharides (5a–5e) were efficiently synthesized with moderate to good yields through glycosylation reaction of various phosphate acceptors, such as dibenzyl phosphate, 6-O-benzyloxyphosphoryl glucoside, 3-O-benzyloxyphosphoryl glucoside, serinyl phosphate and 5′-uridylic acid. To our delight, the exposure of perbenzylated 2-glucosyloxy tropone donor and diphenyl phosphate to visible-light irradiation in the absence of the additives led to a 91% yield of glycosyl phosphate 5f with high α-stereoselectivity (α/β > 20/1). Different glycosyl donors (galactosyl, mannosyl, xylosyl, and 2-azido-2-deoxy-glucosyl donors) effectively reacted under visible-light irradiation, generating the α-glycosyl phosphate 5g–5k with good yields (57–85%). Notably, good stereoselectivity was achieved for the formation of α-diglycosyl phosphate 5l (phosphoric acid as the acceptor). The above reaction was also effective for pyranose-6/2/4-O-phosphate to generate α-glycosyl phosphosaccharide 5m–5p with high anomeric selectivity and good yields by changing different additives. Furthermore, serine-O-phosphate and 5′-uridylic acid were suitable glycosyl acceptors to generate the corresponding α-glycosyl phosphates 5q–5r with high anomeric selectivity.
A Stereoselective synthesis of the glycosyl phosphates and glycosyl phosphosaccharides. B Synthesis of N-glycosides. a Reaction conditions unless otherwise specified: NaOTf (10–50 mol%), ClCH2CH2Cl (2.0 mL), blue LEDs (15 W), argon atmosphere, 2–12 h. b ClCH2CH2Cl (2.0 mL), blue LEDs (15 W), argon atmosphere, 0.5–4 h. Yields of α/β mixtures (α/β mixtures were not separable), and ratios were determined by 31P NMR. c NaSO4Me (30 mol%), blue LEDs (15 W), 1.5–8 h. d blue LEDs (15 W), 100 °C, 10–14 h. e Bis(trimethylsilyl)trifluoroacetamide (BSTFA, 4.0 equiv), ClCH2CH2Cl: CH3CN = 1:1 (1.0 mL: 1.0 mL), argon atmosphere, 0.5 h; then TMSOTf (10 mol%), blue LEDs (15 W), 0.5–2 h, isolated yield.
The substrate scope of photo-N-glycosylation
Our synthetic method was further demonstrated through N-glycosylation (Fig. 3B), which is another important aspect of evaluating the effectiveness of a new glycosyl donor64,65,66. After the pre-trimethylsilylation of pyrimidines with bis(trimethylsilyl)trifluoroacetamide (BSTFA) to improve the solubility of pyrimidines, we observed that pyranosyl-/furanosyl-donors, including D-galacto-, D-gluco-, D-arabino-, and D-ribo-, effectively reacted with pyrimidines to form the corresponding N-glycosides 6a–6d with excellent yields (94-98%). Moreover, the N-glycosylation effectively proceeded with purines, such as 6-chloro-2-fluoropurine, 2,6-dichloropurine, and 6-chloropurine, resulting in satisfactory yields (90–95%) of N-9 nucleoside 6e–6h. All these results showed that 2-glycosyloxy tropones are suitable donors for N-glycosylation.
Late-stage glycosylation and gram-scale synthesis
As late-stage glycosylation is desirable in modern drug discovery3,67, glycosylation of several natural products and pharmaceuticals was evaluated on a gram-scale (Fig. 4A). The mono/di-glycosylation of perbenzoylated 2-galactosyloxy tropone (1c) with epiandrosterone, estradiol benzoate, β-estradiol, and cholesterol generated the corresponding glycosides 7a–7d (1.20–1.70 g) with 80–95% yields. Moreover, glycosylation of perbenzoylated 2-L-rhamnosyloxy tropone (1h) with Simvastatin yielded 94% of gram-scale glycoside 7e. Furthermore, α-glycosyl phosphate 5f and nucleoside 7f were obtained via the visible-light-promoted glycosylation on a gram-scale with excellent stereoselectivity and good yields. These successful applications strongly demonstrate the high practicability and the ease of scalability of our methodology.
A Gram-scale synthesis and modification. Conditions A: TMSOTf (10 mol%), ClCH2CH2Cl, blue LEDs (15 W), 2–24 h, argon atmosphere, isolated yield. Conditions B: ClCH2CH2Cl, blue LEDs (15 W), 2 h, argon atmosphere; Yields of α/β mixtures (α/β mixtures were not separable), and ratios were determined by 31P NMR. Conditions C TMSOTf (10 mol%), BSTFA (4.0 equiv), ClCH2CH2Cl: CH3CN = 1:1, blue LEDs (15 W), 1 h, argon atmosphere, isolated yield. B Iterative synthesis of hexasaccharide. Reaction conditions: (a) TMSOTf (10 mol%), ClCH2CH2Cl, blue LEDs (15 W), argon atmosphere; b TBAF in THF (1 mol/L, 1.5 equiv), THF (2.0 mL).
Interesting, 2-glucosyloxy tropone can indeed serve as a glycosyl acceptor in the presence of molecular sieves, making it a valuable tool for orthogonal glycosylation reactions. 2-glucosyloxy tropone bearing a free hydroxyl group at C-6 (S27 in the Supplementary Information) could undergo glycosylation reactions with thioglycoside donor or glycosyl imidate donor, resulting in the formation of disaccharide products (S29 and S31 in the Supplementary Information) with moderate to good yields.
Hexasaccharide synthesis
The ultimate aim of a glycosylation method is to synthesize glycans4,5,6,18,19,20,21. Thus, a hexasaccharide 8i was prepared via iteratively visible-light-promoted glycosylation (Fig. 4B). O-glycosylation of 2-glucosyloxy tropone 1n with the acceptor 2b conducted under the standard condition yielded 97% of the disaccharide 8a. The deprotection of the TBDPS group used stoichiometric amounts of tetrabutylammonium fluoride to generate the acceptor 8b. The desired hexasaccharide 8i was finally obtained via another four photo-glycosylation/desilylation cycles in a satisfactory isolated yield.
Mechanistic studies
To gain more insight into visible-light-promoted glycosylation, we conducted a series of mechanistic experiments. The model reaction and control experiments are presented in Fig. 5 and Table 1. The experiment results revealed the significant impact of visible light irradiation on the glycosylation reaction. The glycosylation reaction time was significantly reduced when triflate was used to catalyze the reaction. UV−Vis absorption and photoluminescence spectra of 1a revealed that the donor can be excited effectively by absorbing visible light (Fig. 5A, B, Supplementary Figs. 1–2)52,53. The light on-and-off experiments revealed that the glycosylation reaction occurred only under photo-irradiation, indicating that light played an important role in this reaction (Fig. 5C, Supplementary Table 7, Supplementary Figs. 4–5). Tropolone, originating from the leaving group departure, could be isolated as the byproduct68,69. The stereochemical outcome of the glycosyl phosphosaccharides might be attributed to the continuous generation of a highly reactive and low concentration of β-glycosyl triflate70. Consequently, a mechanism involving direct excitation of the donor, aromatic resonance, departure of the leaving group to produce a glycosyl cation, and glycosylation was proposed (Supplementary Fig. 7).
A UV−vis absorption and photoluminescence spectra of 1a.a B The photoluminescence excitation spectra of 1a.a C Light on-and-off experiments using the glycosylation of 1a with 2a. b [a] 0.10 mg/ mL of 1a in ClCH2CH2Cl. [b] 1a (1.5 equiv.), 2a (1.0 equiv.), 1,3,5-trimethoxybenzene, ClCH2CH2Cl, blue LEDs (15 W), yields were determined by 1H NMR.
Discussion
In summary, we developed a versatile visible-light-driven glycosylation method using the 2-glycosyloxy tropone donor. This method was highly effective under the direct visible-light activation of the tropolone-leaving group at ambient temperature without a photosensitizer or activator. In addition, the photo-mediated protocol proved effective in enabling both the O-glycosylation and the N-glycosylation under mild conditions. The glycosylation method can accommodate a wide range of functionally complex acceptors with good scopes, providing a practical approach to glycoside synthesis. Particularly, the reaction demonstrated high versatility with a wide range of phosphates, thus generating glycosyl phosphates/phosphosaccharides with excellent anomeric selectivity. This method is not only suitable for the late-stage glycosylation of natural products and pharmaceuticals on gram scales but also for the iterative synthesis of hexasaccharide. The efficiency, robustness, and selectivity of the presented method make it an attractive protocol for synthesizing glycans and glycoconjugates.
Methods
General procedure for O-glycosylation
The glycosyl donor 1 (0.075 mmol), acceptor 2 (0.05 mmol) and additive (0.005 mmol) were dissolved in dry ClCH2CH2Cl (2.0 mL). The mixture was irradiated by blue LEDs at ambient temperature for 0.5 h. Upon completion, the solvent was concentrated under reduced pressure. The resulting residue was eluted by flash column chromatography (petroleum ether/EtOAc) to afford the glycosylated product 3, 4, 7 and 8.
General Procedure for glycosyl phosphosaccharides
The glycosyl donor 1 (0.06 mmol), acceptor 2 (0.05 mmol) and additive were dissolved in dry ClCH2CH2Cl (2.0 mL). The mixture was irradiated by blue LEDs at ambient temperature. Upon completion, Et3N was added to quench the reaction and the solvent was concentrated under reduced pressure. The resulting residue was eluted by flash column chromatography (petroleum ether/EtOAc) to afford the glycosylated product 5.
General Procedure for N-glycosylation
To a solution of purines or pyrimidines (0.05 mmol) in anhydrous CH3CN (1.0 mL) was added BSTFA (0.20 mmol) under Ar atmosphere. The suspension was stirred at room temperature until it becomes a clear solution. Then the 1 (0.06 mmol) dissolved in ClCH2CH2Cl (1.0 mL) and additive (0.005 mmol) were added to the above solution through a syringe, and the mixture was irradiated by blue LEDs at ambient temperature. Upon completion, the reaction mixture was concentrated in vacuo. The residue was purified by flash column chromatography on silica gel (petroleum ether/EtOAc) to obtain 6.
Data availability
The data supporting the findings of this study are available described in the article and its Supplementary Information Files are available from the corresponding authors on request.
References
Varki, A. Biological roles of glycans. Glycobiology 27, 3–49 (2017).
Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 21, 729–749 (2020).
Shivatare, S. S., Shivatare, V. S. & Wong, C.-H. Glycoconjugates: synthesis, functional studies, and therapeutic developments. Chem. Rev. 122, 15603–15671 (2022).
Demchenko, A. V. Handbook of Chemical Glycosylation: Advances in Stereoselectivity and Therapeutic Relevance (Wiley-VCH, Weinheim, 2008).
Hung, S. C. & Zulueta, M. M. L. Glycochemical Synthesis: Strategies and Applications (First ed., Wiley, Hoboken, 2016).
Bennett, C. S. Selective Glycosylations: Synthetic Methods and Catalysts: Synthetic Methods and Catalysts (Wiley-VCH, Weinheim, 2017).
Singh, Y., Geringer, S. A. & Demchenko, A. V. Synthesis and glycosidation of anomeric halides: evolution from early studies to modern methods of the 21st century. Chem. Rev. 122, 11701–11758 (2022).
Geng, Y. et al. Cooperative catalysis in glycosidation reactions with O-glycosyl trichloroacetimidates as glycosyl donors. Angew. Chem. Int. Ed. 52, 10089–10092 (2013).
Codée, J. D. C., Litjens, R. E. J. N., van den Bos, L. J., Overkleeft, H. S. & van der Marel, G. A. Thioglycosides in sequential glycosylation strategies. Chem. Soc. Rev. 34, 769–782 (2005).
Plante, O. J., Palmacci, E. R., Andrade, R. B. & Seeberger, P. H. Oligosaccharide synthesis with glycosyl phosphate and dithiophosphate triesters as glycosylating agents. J. Am. Chem. Soc. 123, 9545–9554 (2001).
Danishefsky, S. J. & Bilodeau, M. T. Glycals in organic synthesis: the evolution of comprehensive strategies for the assembly of oligosaccharides and glycoconjugates of biological consequence. Angew. Chem. Int. Ed. 35, 1380–1419 (1996).
Ma, X. et al. L. A “traceless” directing group enables catalytic SN2 glycosylation toward 1,2-cis-glycopyranosides. J. Am. Chem. Soc. 143, 11908–11913 (2021).
Hu, Z., Tang, Y. & Yu, B. Glycosylation with 3,5-dimethyl-4-(2′-phenylethynylphenyl) phenyl (EPP) glycosides via a dearomative activation mechanism. J. Am. Chem. Soc. 141, 4806–4810 (2019).
Garcia, B. A., Poole, J. L. & Gin, D. Y. Direct glycosylations with 1-hydroxy glycosyl donors using trifluoromethanesulfonic anhydride and diphenyl sulfoxide. J. Am. Chem. Soc. 119, 7597–7598 (1997).
Tanaka, M. et al. Boronic-acid-catalyzed regioselective and 1,2-cis-stereoselective glycosylation of unprotected sugar acceptors via SNi-type mechanism. J. Am. Chem. Soc. 140, 3644–3651 (2018).
Shu, P. et al. Interrupted pummerer reaction in latent-active glycosylation: glycosyl donors with a recyclable and regenerative leaving group. Angew. Chem. Int. Ed. 54, 14432–14436 (2015).
Zhang, C. et al. Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation. Nat. Chem. 14, 686–694 (2022).
Wu, Y., Xiong, D.-C., Chen, S.-C., Wang, Y.-S. & Ye, X.-S. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat. Commun. 8, 14851 (2017).
Joseph, A. A., Pardo-Vargas, A. & Seeberger, P. H. Total synthesis of polysaccharides by automated glycan assembly. J. Am. Chem. Soc. 142, 8561–8564 (2020).
Zhu, Q. et al. Chemical synthesis of glycans up to a 128-mer relevant to the O-antigen of Bacteroides vulgatus. Nat. Commun. 11, 4142 (2020).
Yao, W. et al. Automated solution-phase multiplicative synthesis of complex glycans up to a 1,080-mer. Nat. Synth. 1, 854–863 (2022).
Liu, Q. & Wu, L.-Z. Recent advances in visible-light-driven organic reactions. Nat. Sci. Rev. 4, 359–380 (2017).
Silvi, M. & Melchiorre, P. Enhancing the potential of enantioselective organocatalysis with light. Nature 554, 41–49 (2018).
Glaser, F., Kerzig, C. & Wenger, O. S. Multi-Photon excitation in photoredox catalysis: concepts, applications,. methods. Angew. Chem. Int. Ed. 59, 10266–10284 (2020).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C−H functionalization reactions. Chem. Rev. 122, 1925–2016 (2022).
Andrews, R. S., Becker, J. J. & Gagné, M. R. Intermolecular addition of glycosyl halides to alkenes mediated by visible light. Angew. Chem. Int. Ed. 49, 7274–7276 (2010).
Wang, Q. et al. Visible light activation enables desulfonylative cross-coupling of glycosyl sulfones. Nat. Synth. 1, 967–974 (2022).
Wei, Y., Wang, Q. & Koh, M. J. A photoinduced, nickel-catalyzed reaction for the stereoselective assembly of C-linked glycosides and glycopeptides. Angew. Chem. Int. Ed. 62, e202214247 (2023).
Xie, D., Wang, Y., Zhang, X., Fu, Z. & Niu, D. Alkyl/Glycosyl sulfoxides as radical precursors and their use in the synthesis of pyridine derivatives. Angew. Chem. Int. Ed. 61, e202204922 (2022).
Hashimoto, S., Kurimoto, I., Fujii, Y. & Noyori, R. Novel nucleophilic substitution reaction by radical cation intermediates. Photosensitized transacetalization via SON1 mechanism. J. Am. Chem. Soc. 107, 1427–1429 (1985).
Cumptsey, I. & Crich, D. Photoinitiated glycosylation at 350 nm. J. Carbohydr. Chem. 30, 469–485 (2011).
Iwata, R., Uda, K., Takahashi, D. & Toshima, K. Photo-induced glycosylation using reusable organophotoacids. Chem. Commun. 50, 10695–10698 (2014).
Mao, R.-Z. et al. Photoinduced C-S bond cleavage of thioglycosides and glycosylation. Org. Lett. 17, 5606–5609 (2015).
Spell, M., Wang, X., Wahba, A. E., Conner, E. S. & Ragains, J. R. An α-selective, visible light photocatalytic glycosylation of alcohols with selenoglycosides. Carbohydr. Res. 369, 42–47 (2013).
Wever, W. J., Cinelli, M. A. & Bowers, A. A. Visible light mediated activation and O-glycosylation of thioglycosides. Org. Lett. 15, 30–33 (2013).
Mao, R.-Z. et al. Light-driven highly efficient glycosylation reactions. Org. Chem. Front. 3, 737–743 (2016).
Spell, M. L. et al. A visible-light-promoted O-glycosylation with a thioglycoside donor. Angew. Chem. Int. Ed. 55, 6515–6519 (2016).
Yu, Y., Xiong, D.-C., Mao, R. Z. & Ye, X.-S. Visible light photoredox-catalyzed O-sialylation using thiosialoside donors. J. Org. Chem. 81, 7134–7138 (2016).
Wen, P. & Crich, D. Blue light photocatalytic glycosylation without electrophilic additives. Org. Lett. 19, 2402–2405 (2017).
Zhao, G. & Wang, T. Stereoselective synthesis of 2-deoxyglycosides from glycals by visible-light-induced photoacid catalysis. Angew. Chem. Int. Ed. 57, 6120–6124 (2018).
Cao, Y. et al. Visible-light-promoted 3,5-dimethoxyphenyl glycoside activation and glycosylation. Chem. Commun. 57, 10899–10902 (2021).
Zhao, G., Li, J. & Wang, T. Visible-light-induced photoacid catalysis: application in glycosylation with O-glycosyl trichloroacetimidates. Chem. Commun. 57, 12659–12662 (2021).
Liu, K. M. et al. Iterative synthesis of 2-deoxyoligosaccharides enabled by stereoselective visible-light-promoted glycosylation. Angew. Chem. Int. Ed. 61, e202114726 (2022).
Zhu, F. et al. Catalytic and photochemical strategies to stabilized radicals based on anomeric nucleophiles. J. Am Chem. Soc. 142, 11102–11113 (2020).
Wan, L.-Q. et al. Nonenzymatic stereoselective S-glycosylation of polypeptides and proteins. J. Am. Chem. Soc. 143, 11919–11926 (2021).
Sangwan, R. & Mandal, P. K. Recent advances in photoinduced glycosylation: oligosaccharides, glycoconjugates and their synthetic applications. RSC Adv 7, 26256–26321 (2017).
Ye, H., Xiao, C. & Lu, L.-Q. Photo-induced carbohydrate synthesis and modification. Chin. J. Org. Chem. 38, 1897–1906 (2018).
Wang, H., Wu, P. R., Zhao, X., Zeng, J. & Wan, Q. Advances on photo-promoted glycosylation reactions. Acta Chim. Sin. 77, 231–241 (2019).
Zhang, H. Y. et al. Photo-/Electro-driven carbohydrate-based reactions. Prog. Chem. 32, 1804–1823 (2020).
Guo, H., Roman, D. & Beemelmanns, C. Tropolone natural products. Nat. Prod. Rep. 36, 1137–1155 (2019).
Breheret, E. F. & Martin, M. M. Electronic relaxation of troponoids: tropolone fluorescence. J. Lumin. 17, 49–60 (1978).
Shoute, L. C. T. et al. Spectroscopy and photophysics of tropolone in condensed media. Phys. Chem. Chem. Phys. 2, 1–9 (2000).
Coote, S. C. 4-π-Photocyclization: scope and synthetic applications. Eur. J. Org. Chem. 1405–1423 (2020).
Ge, Z.-P. et al. Cephalodiones A–D: compound characterization and semisynthesis by [6+6] cycloaddition. Angew. Chem. Int. Ed. 60, 9374–9378 (2021).
Lyons, D. J. M. et al. Tropolonate salts as acyl-transfer catalysts under thermal and photochemical conditions: reaction scope and mechanistic insights. ACS Catal 10, 12596–12606 (2020).
Crich, D. Mechanism of a chemical glycosylation reaction. Acc. Chem. Res. 43, 1144–1153 (2010).
Mootoo, D. R., Konradsson, P., Udodong, U. & Fraser-Reid, B. “Armed” and “Disarmed” -pentenyl glycosides in saccharide couplings leading to oligosaccharides. J. Am. Chem. Soc 110, 5583–5584 (1988).
Kitamura, K., Ando, Y., Matsumoto, T. & Suzuki, K. Total synthesis of aryl C-glycoside natural products: strategies and tactics. Chem. Rev. 118, 1495–1598 (2018).
Xiong, D.-C., Zhang, L.-H. & Ye, X.-S. Bromodimethylsulfonium bromide-silver triflate: a new powerful promoter system for the activation of thioglycosides. Adv. Synth. Catal. 350, 1696–1700 (2008).
Simpson, B. W. & Trent, M. S. Pushing the envelope: LPS modifications and their consequences. Nat. Rev. Microbiol. 17, 403–416 (2019).
Hanessian, S., Lu, P.-P. & Ishida, H. One-Step, stereocontrolled synthesis of glycosyl 1-phosphates, uridine-5′-diphosphogalactose, and uridine-5′-diphosphoglucose from unprotected glycosyl donors. J. Am. Chem. Soc. 120, 13296–13300 (1998).
Zhang, X. et al. Stereoselective gold(I)-catalyzed approach to the synthesis of complex α-glycosyl phosphosaccharides. Nat. Commun. 13, 421 (2022).
Tanaka, H., Iwata, Y., Takahashi, D., Adachi, M. & Takahashi, T. Efficient stereoselective synthesis of γ-N-glycosyl asparagines by N-glycosylation of primary amide groups. J. Am. Chem. Soc. 127, 1630–1631 (2005).
Zhang, Q., Sun, J., Zhu, Y., Zhang, F. & Yu, B. An efficient approach to the synthesis of nucleosides: gold(I)-catalyzed N-glycosylation of pyrimidines and purines with glycosylortho-alkynyl benzoates. Angew. Chem. Int. Ed. 50, 4933–4936 (2011).
Li, P. et al. Glycosyl ortho-(1-phenylvinyl)benzoates versatile glycosyl donors for highly efficient synthesis of both O-glycosides and nucleosides. Nat. Commun. 11, 405 (2020).
Moradi, S. V., Hussein, W. M., Varamini, P., Simerska, P. & Toth, I. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem. Sci. 7, 2492–2500 (2016).
Lyons, D. J., Crocker, R. D., Blumel, M. & Nguyen, T. V. Promotion of organic reactions by non-benzenoid carbocyclic aromatic ions. Angew. Chem. Int. Ed. 56, 1466–1484 (2017).
Lyons, D. J. M., Crocker, R. D., Enders, D. & Nguyen, T. V. Tropylium salts as efficient organic Lewis acid catalysts for acetalization and transacetalization reactions in batch and flow. Green Chem 19, 3993–3996 (2017).
Adero, P. O., Amarasekara, H., Wen, P., Bohé, L. & Crich, D. The experimental evidence in support of glycosylation mechanisms at the SN1–SN2 interface. Chem. Rev. 118, 8242–8284 (2018).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 21977003), the National Key R&D Program of China (No. 2022YFC3400800), and the Fundamental Research Funds for the Central Universities. We are grateful to the following Professors: Houhua Li, Xinjing Tang, Song Song, and Suwei Dong, for their insightful discussions.
Author information
Authors and Affiliations
Contributions
D.-C.X. conceived the research. J.Z. performed the main experiments. Z-X.L., X.W., C-F.G., P-Y.W., J-Z.C., and M.L., M.L. synthesized some building blocks. D.-C.X. and J.Z. analyzed the data. D.-C.X., J.Z., and X.-S.Y. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Luo, ZX., Wu, X. et al. Photosensitizer-free visible-light-promoted glycosylation enabled by 2-glycosyloxy tropone donors. Nat Commun 14, 8025 (2023). https://doi.org/10.1038/s41467-023-43786-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-43786-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.