Featured
-
-
Article
| Open Access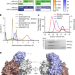 Structure of the MRAS–SHOC2–PP1C phosphatase complex
Structure of the MRAS–SHOC2–PP1C phosphatase complex
A structure of the MRAS–SHOC2–PP1C complex supports a RAS-driven and multi-molecular model for RAF activation in which individual RAS–GTP molecules recruit RAF–14-3-3 and SHOC2–PP1C to activate the downstream pathway.
- Zachary J. Hauseman
- , Michelle Fodor
- & Daniel A. King
-
Article
| Open Access Reconstitution of a telomeric replicon organized by CST
Reconstitution of a telomeric replicon organized by CST
The Polα–primase-associated CST complex organizes telomeric C-strand DNA synthesis, and, in combination with telomerase, it carries out complete replication of the single-stranded DNA overhang found at human telomeres.
- Arthur J. Zaug
- , Karen J. Goodrich
- & Thomas R. Cech
-
Article |
 Structures of the human CST-Polα–primase complex bound to telomere templates
Structures of the human CST-Polα–primase complex bound to telomere templates
A structural analysis demonstrates how the single-stranded DNA-binding accessory protein complex CST physically organizes the human DNA polymerase-α–primase complex for efficient primer synthesis during telomere replication.
- Qixiang He
- , Xiuhua Lin
- & Ci Ji Lim
-
Article
| Open Access Biosynthesis of strychnine
Biosynthesis of strychnine
The biosynthetic pathway of strychnine, brucine and diaboline is described, and the biosynthesis of these complex, pharmacologically active compounds has been successfully recapitulated in Nicotiana benthamiana from an upstream intermediate.
- Benke Hong
- , Dagny Grzech
- & Sarah E. O’Connor
-
Article
| Open Access A male steroid controls female sexual behaviour in the malaria mosquito
A male steroid controls female sexual behaviour in the malaria mosquito
The discovery of a male-specific sex hormone in the mosquito <em>Anopheles gambiae</em> may allow new strategies for the control of this notorious disease vector.
- Duo Peng
- , Evdoxia G. Kakani
- & Flaminia Catteruccia
-
Article
| Open Access GTSF1 accelerates target RNA cleavage by PIWI-clade Argonaute proteins
GTSF1 accelerates target RNA cleavage by PIWI-clade Argonaute proteins
The evolutionarily conserved RNA-binding protein GTSF1 and its homologues interact with members of the PIWI class of Argonaute proteins, increasing the efficiency of the RNA-cleaving activity of PIWI proteins, an essential function across the animal kingdom.
- Amena Arif
- , Shannon Bailey
- & Phillip D. Zamore
-
Article
| Open Access Structural basis for SHOC2 modulation of RAS signalling
Structural basis for SHOC2 modulation of RAS signalling
Cryo-electron microscopy structure, molecular dynamics and biochemical analyses of the SHOC2–PP1C–MRAS complex demonstrate the dependence of the complex formation on RAS–GTP and identify the determinants of RAS isoform preference for SHOC2–PP1C and specificity of the complex for RAF dephosphorylation.
- Nicholas P. D. Liau
- , Matthew C. Johnson
- & Jawahar Sudhamsu
-
Article
| Open Access Structural insights into dsRNA processing by Drosophila Dicer-2–Loqs-PD
Structural insights into dsRNA processing by Drosophila Dicer-2–Loqs-PD
Structures of the Dcr-2–Loqs-PD complex while it is processing a double-stranded RNA (dsRNA) substrate elucidate the interactions between Dcr-2 and Loqs-PD, and show that Dcr-2 undergoes substantial conformational changes during a dsRNA-processing cycle.
- Shichen Su
- , Jia Wang
- & Jinbiao Ma
-
Article
| Open Access Structure of the Dicer-2–R2D2 heterodimer bound to a small RNA duplex
Structure of the Dicer-2–R2D2 heterodimer bound to a small RNA duplex
Cryo-electron microscopy structures of Drosophila Dicer-2–R2D2 complexes with and without small interfering RNA reveal how the RNA is presented to Argonaute in the correct orientation for viral gene silencing.
- Sonomi Yamaguchi
- , Masahiro Naganuma
- & Osamu Nureki
-
Article |
 eIF5B and eIF1A reorient initiator tRNA to allow ribosomal subunit joining
eIF5B and eIF1A reorient initiator tRNA to allow ribosomal subunit joining
Single-molecule spectroscopy and structural studies were used to examine the dynamics of association of eIF1A and eIF5B with the human translation initiation complex and their role in presenting tRNA to the complex to initiate translation.
- Christopher P. Lapointe
- , Rosslyn Grosely
- & Joseph D. Puglisi
-
Article
| Open Access Mechanism of replication origin melting nucleated by CMG helicase assembly
Mechanism of replication origin melting nucleated by CMG helicase assembly
Cryo-electron microscopy structures of the sequential assembly of the CMG replicative helicase on a chromatinized origin of replication provide insights into the mechanism through which DNA melting is initiated by ATP binding.
- Jacob S. Lewis
- , Marta H. Gross
- & Alessandro Costa
-
Article |
 Deciphering the immunopeptidome in vivo reveals new tumour antigens
Deciphering the immunopeptidome in vivo reveals new tumour antigens
A newly developed genetically engineered mouse model enables the analysis of specific antigen presentation in vivo, providing insights into the tumour immunopeptidome and cancer progression.
- Alex M. Jaeger
- , Lauren E. Stopfer
- & Tyler Jacks
-
Article |
 An exercise-inducible metabolite that suppresses feeding and obesity
An exercise-inducible metabolite that suppresses feeding and obesity
A newly identified exercise-induced signalling metabolite—an amidated conjugate of lactate and phenylalanine—can reduce food intake and improve blood glucose homeostasis.
- Veronica L. Li
- , Yang He
- & Jonathan Z. Long
-
Research Briefing |
 A hybrid control model for the eukaryotic cell cycle
A hybrid control model for the eukaryotic cell cycle
The organizational principles of the eukaryotic cell cycle have yet to be pinned down, and two opposing models have been put forward. Genetic and proteomics analyses in a model eukaryote, fission yeast, reveal that the cell cycle is organized through a hybrid of both models, although the contribution of one strongly outweighs the other.
-
Article
| Open Access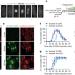 Core control principles of the eukaryotic cell cycle
Core control principles of the eukaryotic cell cycle
The core cell cycle is largely driven by increasing total CDK activity together with minor differences in the substrate specificity of the CDKs initiating DNA replication and mitosis.
- Souradeep Basu
- , Jessica Greenwood
- & Paul Nurse
-
Article |
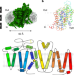 Structural basis of GABA reuptake inhibition
Structural basis of GABA reuptake inhibition
Structural determination of GAT1 using cryo-electron microscopy provides insights into the biology and pharmacology of this GABA transporter.
- Zenia Motiwala
- , Nanda Gowtham Aduri
- & Cornelius Gati
-
Article
| Open Access ATGL is a biosynthetic enzyme for fatty acid esters of hydroxy fatty acids
ATGL is a biosynthetic enzyme for fatty acid esters of hydroxy fatty acids
A study in mammals identifies a new role for adipose triglyceride lipase in catalysing the esterification of hydroxyl fatty acids to produce biologically active fatty acid esters of hydroxy fatty acids.
- Rucha Patel
- , Anna Santoro
- & Barbara B. Kahn
-
Article
| Open Access Mechanism of mitoribosomal small subunit biogenesis and preinitiation
Mechanism of mitoribosomal small subunit biogenesis and preinitiation
Structural analysis of several small mitoribosomal subunit intermediates reveals a sequential mechanism of biogenesis, and how assembly links to initiation to form active mitoribosomes.
- Yuzuru Itoh
- , Anas Khawaja
- & Alexey Amunts
-
Article
| Open Access Discovery of non-squalene triterpenes
Discovery of non-squalene triterpenes
Chimeric triterpene synthases are identified that catalyse non-squalene-dependent triterpene biosynthesis.
- Hui Tao
- , Lukas Lauterbach
- & Tiangang Liu
-
News & Views |
 Sinking diatoms trap silicon in deep seawater of acidified oceans
Sinking diatoms trap silicon in deep seawater of acidified oceans
The seas are acidifying as a result of carbon dioxide emissions. It now emerges that this will alter the solubility of the shells of marine organisms called diatoms — and thereby change the distribution of nutrients and plankton in the ocean.
- David A. Hutchins
-
Article |
 Defining mitochondrial protein functions through deep multiomic profiling
Defining mitochondrial protein functions through deep multiomic profiling
A multiomics resource characterizing human mitochondrial proteins enables identification of biological functions and supports genetic diagnosis of mitochondrial pathologies.
- Jarred W. Rensvold
- , Evgenia Shishkova
- & David J. Pagliarini
-
Article |
 Intracellular lipid surveillance by small G protein geranylgeranylation
Intracellular lipid surveillance by small G protein geranylgeranylation
In Caenorhabditis elegans, geranylgeranyl conjugation to RAB-11.1 acts as a lipid sensor to regulate nutrient uptake and lipid metabolism in response to metabolic demand.
- Abigail Watterson
- , Lexus Tatge
- & Peter M. Douglas
-
Article
| Open Access Organocatalytic stereoselective cyanosilylation of small ketones
Organocatalytic stereoselective cyanosilylation of small ketones
The development of confined organocatalysts for the enantioselective cyanosilylation of small, unbiased substrates, including 2-butanone, is shown to lead to catalysts that are as selective as enzymes, with excellent levels of control.
- Hui Zhou
- , Yu Zhou
- & Benjamin List
-
Article |
 Molecular basis for the initiation of DNA primer synthesis
Molecular basis for the initiation of DNA primer synthesis
The molecular determinants for primer synthesis are identified within the catalytic domain of primase-polymerase enzymes, elucidating the mechanisms underlying initiation of primer synthesis.
- Arthur W. H. Li
- , Katerina Zabrady
- & Aidan J. Doherty
-
Article |
 Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA
Selective inhibition of miRNA processing by a herpesvirus-encoded miRNA
Herpesvirus microRNAs interfere directly with host cell microRNA processing, thereby disrupting mitochondrial architecture, evading intrinsic host defences and driving the switch from latent to lytic infection.
- Thomas Hennig
- , Archana B. Prusty
- & Bhupesh K. Prusty
-
Article
| Open Access USP14-regulated allostery of the human proteasome by time-resolved cryo-EM
USP14-regulated allostery of the human proteasome by time-resolved cryo-EM
Structures of the human ubiquitin-specific protease 14 in complex with the 26S proteasome captured in the act of protein degradation provide a detailed view of the functional cycle of the USP14-regulated proteasome.
- Shuwen Zhang
- , Shitao Zou
- & Youdong Mao
-
Matters Arising |
 Evaluating ribosomal frameshifting in CCR5 mRNA decoding
Evaluating ribosomal frameshifting in CCR5 mRNA decoding
- Yousuf A. Khan
- , Gary Loughran
- & John F. Atkins
-
Article |
 Structure of active human telomerase with telomere shelterin protein TPP1
Structure of active human telomerase with telomere shelterin protein TPP1
Cryo-electron microscopy structures of human telomerase and telomerase in complex with TPP1 provide insights into the interactions of these proteins and their activities.
- Baocheng Liu
- , Yao He
- & Juli Feigon
-
Article |
 Structural basis for the tethered peptide activation of adhesion GPCRs
Structural basis for the tethered peptide activation of adhesion GPCRs
Adhesion GPCRs involved in cell and matrix interactions signal through a distinct self-cleavage, self-activation mechanism.
- Yu-Qi Ping
- , Peng Xiao
- & Jin-Peng Sun
-
Article |
 Tethered peptide activation mechanism of the adhesion GPCRs ADGRG2 and ADGRG4
Tethered peptide activation mechanism of the adhesion GPCRs ADGRG2 and ADGRG4
Cryo-electron microscopy structures of three adhesion G protein-coupled receptors (aGPCRs) complexes provide insight into the tethered activation mechanism of aGPCRs and show the potential for rational design of agonists.
- Peng Xiao
- , Shengchao Guo
- & Xiao Yu
-
Article |
 The tethered peptide activation mechanism of adhesion GPCRs
The tethered peptide activation mechanism of adhesion GPCRs
Cryo-electron microscopy structures of GPR56 and latrophilin 3 show how the released tethered agonist peptide interacts with the transmembrane domain, suggesting a model for the activation mechanism of adhesion G-protein-coupled receptors.
- Ximena Barros-Álvarez
- , Robert M. Nwokonko
- & Georgios Skiniotis
-
Article
| Open Access Structural basis of tethered agonism of the adhesion GPCRs ADGRD1 and ADGRF1
Structural basis of tethered agonism of the adhesion GPCRs ADGRD1 and ADGRF1
Cryo-electron microscopy structures of the adhesion G protein-coupled receptors ADGRD1 and ADGRF1 provide insight into how these receptors are activated in an intrinsic manner through a ‘stalk’ region that acts as a tethered agonist.
- Xiangli Qu
- , Na Qiu
- & Beili Wu
-
Article
| Open Access Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity
Phage anti-CBASS and anti-Pycsar nucleases subvert bacterial immunity
A study using a biochemical screen of 57 phages in two bacterial species identifies and characterizes proteins enabling phages to evade CBASS and Pycsar immune systems, and describes the mechanisms involved.
- Samuel J. Hobbs
- , Tanita Wein
- & Philip J. Kranzusch
-
Article |
 Structural basis of lipopolysaccharide maturation by the O-antigen ligase
Structural basis of lipopolysaccharide maturation by the O-antigen ligase
Cryo-electron microscopy structures of the bacterial O-antigen ligase WaaL, combined with genetics, biochemistry and molecular dynamics simulations, provide insight into the mechanism by which WaaL catalyses the biosynthesis of lipopolysaccharide.
- Khuram U. Ashraf
- , Rie Nygaard
- & Filippo Mancia
-
Article |
 Multifunctional biocatalyst for conjugate reduction and reductive amination
Multifunctional biocatalyst for conjugate reduction and reductive amination
A biocatalytic enzyme originating from bacteria, EneIRED, facilitates amine-activated conjugate alkene reduction followed by reductive amination, efficiently preparing chiral amine diastereomers, which are commonly used in pharmaceuticals and agrochemicals.
- Thomas W. Thorpe
- , James R. Marshall
- & Nicholas J. Turner
-
Article |
 Basis of narrow-spectrum activity of fidaxomicin on Clostridioides difficile
Basis of narrow-spectrum activity of fidaxomicin on Clostridioides difficile
Structural analysis of Clostridioides difficile RNA polymerase in complex with fidaxomicin combined with biochemical, genetic and bioinformatic analyses identifies a key residue that determines fidaxomicin sensitivity.
- Xinyun Cao
- , Hande Boyaci
- & Elizabeth A. Campbell
-
Article
| Open Access Rixosomal RNA degradation contributes to silencing of Polycomb target genes
Rixosomal RNA degradation contributes to silencing of Polycomb target genes
The rixosome associates with Polycomb repressive complexes and chromatin and has a role in silencing of Polycomb target gene expression in human cells via degradation of nascent RNA transcripts.
- Haining Zhou
- , Chad B. Stein
- & Danesh Moazed
-
Article |
 Structure, substrate recognition and initiation of hyaluronan synthase
Structure, substrate recognition and initiation of hyaluronan synthase
A cryo-electron microscopy analysis reveals how HAS selects its substrates, hydrolyses the first substrate to prime the synthesis reaction, opens a hyaluronan-conducting transmembrane channel, ensures alternating substrate polymerization and coordinates hyaluronan inside its transmembrane pore.
- Finn P. Maloney
- , Jeremi Kuklewicz
- & Jochen Zimmer
-
Article
| Open Access Activation mechanism of the class D fungal GPCR dimer Ste2
Activation mechanism of the class D fungal GPCR dimer Ste2
Cryo-electron microscopy structures of ligand-free, agonist-bound and antagonist-bound Ste2 show that this class D1 G protein-coupled receptor has a distinct mechanism of activation compared with other receptor classes.
- Vaithish Velazhahan
- , Ning Ma
- & Christopher G. Tate
-
News & Views |
 Methane might be made by all living organisms
Methane might be made by all living organisms
It is textbook knowledge that some bacteria can generate methane enzymatically. A study now provides evidence that an alternative, non-enzymatic mode of methane production could occur in all metabolically active cells.
- Chang Liu
- & Jingyao Zhang
-
Article |
 Discovery of a Ni2+-dependent guanidine hydrolase in bacteria
Discovery of a Ni2+-dependent guanidine hydrolase in bacteria
A bacterial enzyme is characterized and demonstrated to have Ni2+-dependent activity and high specificity for free guanidine enabling the bacteria to use guanidine as the sole nitrogen source for growth.
- D. Funck
- , M. Sinn
- & J. S. Hartig
-
Article |
 A non-canonical tricarboxylic acid cycle underlies cellular identity
A non-canonical tricarboxylic acid cycle underlies cellular identity
A non-canonical tricarboxylic acid cycle is required for changes in cell state.
- Paige K. Arnold
- , Benjamin T. Jackson
- & Lydia W. S. Finley
-
Article |
 Methane formation driven by reactive oxygen species across all living organisms
Methane formation driven by reactive oxygen species across all living organisms
Methane formation by a ROS-mediated process is linked to metabolic activity and is identified as a conserved feature across living systems.
- Leonard Ernst
- , Benedikt Steinfeld
- & Frank Keppler
-
Article |
 Structure determination of high-energy states in a dynamic protein ensemble
Structure determination of high-energy states in a dynamic protein ensemble
Combining NMR spectroscopy-derived pseudocontact shifts (PCSs) with Carr–Purcell–Meiboom–Gill (CPMG) relaxation dispersion enables protein structure determination of lowly populated high-energy states that are essential for macromolecular function.
- John B. Stiller
- , Renee Otten
- & Dorothee Kern
-
Research Briefing |
 Determining the structure of fleeting protein states
Determining the structure of fleeting protein states
Proteins adopt unstable, high-energy states that exist for fractions of a second but can have key biological roles. A new method of determining high-resolution structures of such states using a form of nuclear magnetic resonance reveals how small changes in protein shape are essential to their function.
-
Article |
 Biocatalytic oxidative cross-coupling reactions for biaryl bond formation
Biocatalytic oxidative cross-coupling reactions for biaryl bond formation
A study presents a biocatalytic method for the formation of sterically hindered biaryl bonds, providing a tunable approach for assembling molecules with catalyst-controlled reactivity, site selectivity and atroposelectivity.
- Lara E. Zetzsche
- , Jessica A. Yazarians
- & Alison R. H. Narayan
-
Article
| Open Access Structural basis for mismatch surveillance by CRISPR–Cas9
Structural basis for mismatch surveillance by CRISPR–Cas9
Cryo-electron microscopy structures of Cas9 during mismatch cleavage provide insight into the mechanisms that control off-target effects of Cas9, which will aid in the future design of high-fidelity Cas9 variants with reduced off-target cleavage.
- Jack P. K. Bravo
- , Mu-Sen Liu
- & David W. Taylor
-
Article |
 Establishment of fetomaternal tolerance through glycan-mediated B cell suppression
Establishment of fetomaternal tolerance through glycan-mediated B cell suppression
Pathways of glycan-mediated B cell suppression during pregnancy are important for promoting fetomaternal tolerance.
- G. Rizzuto
- , J. F. Brooks
- & A. Erlebacher
-
Article |
 Overcoming universal restrictions on metal selectivity by protein design
Overcoming universal restrictions on metal selectivity by protein design
An alternative approach to metalloprotein design shows that it is possible to overcome the restrictions of the Irving–Williams series and achieve both flexibility and specificity in the binding of metal ions.
- Tae Su Choi
- & F. Akif Tezcan
Browse broader subjects
Browse narrower subjects
- Biocatalysis
- Biogeochemistry
- Bioinorganic chemistry
- Biophysical chemistry
- Carbohydrates
- Chemical modification
- Cytokines
- DNA
- Enzyme mechanisms
- Enzymes
- Glycobiology
- Glycomics
- Histocytochemistry
- Hormones
- Immunochemistry
- Ion channels
- Isoenzymes
- Kinases
- Lipidomics
- Lipids
- Metabolomics
- Metals
- Neurochemistry
- Peptides
- Prions
- Proteases
- Protein folding
- Proteins
- Proteolysis
- Proteomics
- RNA
- Structural biology

 Mechanisms and inhibition of Porcupine-mediated Wnt acylation
Mechanisms and inhibition of Porcupine-mediated Wnt acylation