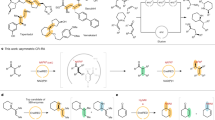
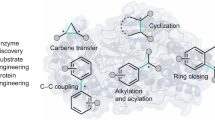
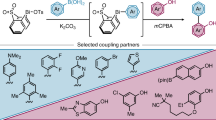
Abstract
Biaryl compounds, with two connected aromatic rings, are found across medicine, materials science and asymmetric catalysis1,2. The necessity of joining arene building blocks to access these valuable compounds has inspired several approaches for biaryl bond formation and challenged chemists to develop increasingly concise and robust methods for this task3. Oxidative coupling of two C–H bonds offers an efficient strategy for the formation of a biaryl C–C bond; however, fundamental challenges remain in controlling the reactivity and selectivity for uniting a given pair of substrates4,5. Biocatalytic oxidative cross-coupling reactions have the potential to overcome limitations inherent to numerous small-molecule-mediated methods by providing a paradigm with catalyst-controlled selectivity6. Here we disclose a strategy for biocatalytic cross-coupling through oxidative C–C bond formation using cytochrome P450 enzymes. We demonstrate the ability to catalyse cross-coupling reactions on a panel of phenolic substrates using natural P450 catalysts. Moreover, we engineer a P450 to possess the desired reactivity, site selectivity and atroposelectivity by transforming a low-yielding, unselective reaction into a highly efficient and selective process. This streamlined method for constructing sterically hindered biaryl bonds provides a programmable platform for assembling molecules with catalyst-controlled reactivity and selectivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Raw data associated with biocatalytic reactions and protein engineering efforts supporting the conclusions of this work are available from the corresponding author upon reasonable request.
References
Yet, L. Privileged Structures in Drug Discovery: Medicinal Chemistry and Synthesis 1st edn, 83–154 (Wiley, 2018).
Yoon, T. P. & Jacobsen, E. N. Privileged chiral catalysts. Science 299, 1691–1693 (2003).
Ashenhurst, J. A. Intermolecular oxidative cross-coupling of arenes. Chem. Soc. Rev. 39, 540–548 (2010).
Kozlowski, M. C. Oxidative coupling in complexity building transforms. Acc. Chem. Res. 50, 638–643 (2017).
Yang, Y., Lan, J. & You, J. Oxidative C–H/C–H coupling reactions between two (hetero)arenes. Chem. Rev. 117, 8787–8863 (2017).
Hüttel, W. & Müller, M. Regio- and stereoselective intermolecular phenol coupling enzymes in secondary metabolite biosynthesis. Nat. Prod. Rep. 38, 1011–1043 (2021).
Lunxiang, Y. & Liebscher, J. Carbon−carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 107, 133–173 (2007).
Boström, J., Brown, D. G., Young, R. J. & Keserü, G. M. Expanding the medicinal chemistry synthetic toolbox. Nat. Rev. Drug Discov. 17, 709–727 (2018).
Yin, J., Rainka, M. P., Zhang, X.-X. & Buchwald, S. L. A highly active Suzuki catalyst for the synthesis of sterically hindered biaryls: novel ligand coordination. J. Am. Chem. Soc. 124, 1162–1163 (2002).
Cammidge, A. N. & Crépy, K. V. L. Synthesis of chiral binaphthalenes using the asymmetric Suzuki reaction. Tetrahedron 60, 4377–4386 (2004).
Martin, R. & Buchwald, S. L. Palladium-catalyzed Suzuki−Miyaura cross-coupling reactions employing dialkylbiaryl phosphine ligands. Acc. Chem. Res. 41, 1461–1473 (2008).
Valente, C. et al. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 51, 3314–3332 (2012).
Patel, N. D. et al. Computationally assisted mechanistic investigation and development of Pd-catalyzed asymmetric Suzuki–Miyaura and Negishi cross-coupling reactions for tetra-ortho-substituted biaryl synthesis. ACS Catal. 8, 10190–10209 (2018).
Ackermann, L., Potukuchi, H. K., Althammer, A., Born, R. & Mayer, P. Tetra-ortho-substituted biaryls through palladium-catalyzed Suzuki−Miyaura couplings with a diaminochlorophosphine ligand. Org. Lett. 12, 1004–1007 (2010).
Brown, D. G. & Boström, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Lee, Y. E., Cao, T., Torruellas, C. & Kozlowski, M. C. Selective oxidative homo- and cross-coupling of phenols with aerobic catalysts. J. Am. Chem. Soc. 136, 6782–6785 (2014).
Nieves-Quinones, Y. et al. Chromium-salen catalyzed cross-coupling of phenols: mechanism and origin of the selectivity. J. Am. Chem. Soc. 141, 10016–10032 (2019).
Shalit, H., Dyadyuk, A. & Pappo, D. Selective oxidative phenol coupling by iron catalysis. J. Org. Chem. 84, 1677–1686 (2019).
Reiss, H. et al. Cobalt(II)[salen]-catalyzed selective aerobic oxidative cross-coupling between electron-rich phenols and 2-naphthols. J. Org. Chem. 84, 7950–7960 (2019).
Röckl, J. L., Schollmeyer, D., Franke, R. & Waldvogel, S. R. Dehydrogenative anodic C−C coupling of phenols bearing electron-withdrawing groups. Angew. Chem. Int. Ed. 59, 315–319 (2020).
Kang, H. et al. Enantioselective vanadium-catalyzed oxidative coupling: development and mechanistic insights. J. Org. Chem. 83, 14362–14384 (2018).
Libman, A. et al. Synthetic and predictive approach to unsymmetrical biphenols by iron-catalyzed chelated radical–anion oxidative coupling. J. Am. Chem. Soc. 137, 11453–11460 (2015).
Morimoto, K., Sakamoto, K., Ohshika, T., Dohi, T. & Kita, Y. Organo-iodine(III)-catalyzed oxidative phenol–arene and phenol–phenol cross-coupling reaction. Angew. Chem. Int. Ed. 55, 3652–3656 (2016).
More, N. Y. & Jeganmohan, M. Oxidative cross-coupling of two different phenols: an efficient route to unsymmetrical biphenols. Org. Lett. 17, 3042–3045 (2015).
Egami, H. & Katsuki, T. Iron-catalyzed asymmetric aerobic oxidation: oxidative coupling of 2-naphthols. J. Am. Chem. Soc. 131, 6082–6083 (2009).
Hovorka, M., Günterova, J. & Zavada, J. Highly selective oxidative cross-coupling of substituted 2-naphthols: a convenient approach to unsymmetrical 1, 1′-binaphthalene-2, 2′-diols. Tetrahedron Lett. 31, 413–416 (1990).
Li, X., Hewgley, J. B., Mulrooney, C. A., Yang, J. & Kozlowski, M. C. Enantioselective oxidative biaryl coupling reactions catalyzed by 1,5-diazadecalin metal complexes: efficient formation of chiral functionalized BINOL derivatives. J. Org. Chem. 68, 5500–5511 (2003).
Tian, J.-M. et al. Copper-complex-catalyzed asymmetric aerobic oxidative cross-coupling of 2-naphthols: enantioselective synthesis of 3,3′-substituted C1-symmetric BINOLs. Angew. Chem. Int. Ed. 58, 11023–11027 (2019).
Bringmann, G. et al. Atroposelective synthesis of axially chiral biaryl compounds. Angew. Chem. Int. Ed. 44, 5384–5427 (2005).
Kočovský, P., Vyskočil, Š. & Smrčina, M. Non-symmetrically substituted 1,1‘-binaphthyls in enantioselective catalysis. Chem. Rev. 103, 3213–3246 (2003).
Kozlowski, M. C., Morgan, B. J. & Linton, E. C. Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling. Chem. Soc. Rev. 38, 3193–3207 (2009).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
Aldemir, H., Richarz, R. & Gulder, T. A. The biocatalytic repertoire of natural biaryl formation. Angew. Chem. Int. Ed. 53, 8286–8293 (2014).
Mate, D. M. & Alcalde, M. Laccase: a multi-purpose biocatalyst at the forefront of biotechnology. Microb. Biotechnol. 10, 1457–1467 (2017).
Sagui, F. et al. Laccase-catalyzed coupling of catharanthine and vindoline: an efficient approach to the bisindole alkaloid anhydrovinblastine. Tetrahedron 65, 312–317 (2009).
Obermaier, S., Thiele, W., Fürtges, L. & Müller, M. Enantioselective phenol coupling by laccases in the biosynthesis of fungal dimeric naphthopyrones. Angew. Chem. Int. Ed. 58, 9125–9128 (2019).
Fasan, R. Tuning P450 enzymes as oxidation catalysts. ACS Catal. 2, 647–666 (2012).
Gil Girol, C. et al. Regio‐ and stereoselective oxidative phenol coupling in Aspergillus niger. Angew. Chem. Int. Ed. 51, 9788–9791 (2012).
Mazzaferro, L. S., Huttel, W., Fries, A. & Müller, M. Cytochrome P450-catalyzed regio- and stereoselective phenol coupling of fungal natural products. J. Am. Chem. Soc. 137, 12289–12295 (2015).
Chakrabarty, S., Wang, Y., Perkins, J. C. & Narayan, A. R. H. Scalable biocatalytic C–H oxyfunctionalization reactions. Chem. Soc. Rev. 49, 8137–8155 (2020).
Noji, M., Nakajima, M. & Koga, K. A new catalytic system for aerobic oxidative coupling of 2-naphthol derivatives by the use of CuCl-amine complex: a practical synthesis of binaphthol derivatives. Tetrahedron Lett. 35, 7983–7984 (1994).
Nakajima, M. Synthesis and application of novel biaryl compounds with axial chirality as catalysts in enantioselective reactions. Yakugaku Zasshi 120, 68–75 (2000).
Langeslay, R. R. et al. Catalytic applications of vanadium: a mechanistic perspective. Chem. Rev. 119, 2128–2191 (2018).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Gerlt, J. A. et al. Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST): a web tool for generating protein sequence similarity networks. Biochim. Biophys. Acta 1854, 1019–1037 (2015).
Zallot, R., Oberg, N. O. & Gerlt, J. A. ‘Democratized’ genomic enzymology web tools for functional assignment. Curr. Opin. Chem. Biol. 47, 77–85 (2018).
Zallot, R., Oberg, N. & Gerlt, J. A. The EFI web resource for genomic enzymology tools: leveraging protein, genome, and metagenome databases to discover novel enzymes and metabolic pathways. Biochemistry 58, 4169–4182 (2019).
Funa, N., Funabashi, M., Ohnishi, Y. & Horinouchi, S. Biosynthesis of hexahydroxyperylenequinone melanin via oxidative aryl coupling by cytochrome P-450 in Streptomyces griseus. J. Bacteriol. 187, 8149–8155 (2005).
Zhao, B. et al. Binding of two flaviolin substrate molecules, oxidative coupling, and crystal structure of Streptomyces coelicolor A3(2) cytochrome P450 158A2. J. Biol. Chem. 280, 11599–11607 (2005).
Li, S., Podust, L. M. & Sherman, D. H. Engineering and analysis of a self-sufficient biosynthetic cytochrome P450 PikC fused to the RhFRED reductase domain. J. Am. Chem. Soc. 129, 12940–12941 (2007).
Acknowledgements
This research was supported by funds from the University of Michigan’s Life Sciences Institute and the Chemistry Department, Alfred P. Sloan Foundation, and Research Corporation Cottrell Scholars programme. Initial studies on the selectivity of dimerization reactions were supported by the National Institutes of Health (NIH; R35 GM124880), and protein engineering (rounds 4–7) and bioinformatic-based library generation were supported by generous funds from the Novartis Global Scholars Program. L.E.Z. is grateful to the NIH National Center for Complementary and Integrative Health (F31AT010973), J.A.Y. thanks the National Science Foundation Graduate Research Fellowship Program, A.L.L. acknowledges the Rackham Graduate School (University of Michigan) and the NIH (F31 NS111906) for funding. We thank M. Müller for supplying the expression vector containing KtnC and D. Sherman for supplying the expression vector containing RhFRed. We thank E. A. Meucci, E. C. Bornowski and J. B. Pyser for assistance with the synthesis of substrates and C.-H. Chiang for help with acquisition of circular dichroism spectra.
Author information
Authors and Affiliations
Contributions
L.E.Z., J.A.Y., S.C., M.E.H. and A.R.H.N. designed experiments and wrote the manuscript with feedback from all authors. L.E.Z. and A.L.L. generated yeast strains used in this work. L.E.Z. performed protein engineering and generated SSNs presented in this work. L.E.Z., J.A.Y., M.E.H. and L.A.M.M. conducted biocatalytic reactions. J.A.Y., S.C. and M.E.H. synthesized substrates and racemic product standards. S.C. carried out product isolation from preparative-scale biocatalytic reactions. L.A.J. calculated circular dichroism spectra and assigned the absolute configuration of products.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Nicholas Turner and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains the following sections: I. Chemical synthesis; II. Protein sequences, expression, and purification; III. Biocatalytic reactions with fungal P450 KtnC; IV. Directed evolution of KtnC; V. Biocatalytic reactions with P450–RhFRed enzymes; VI. Preparative-scale biocatalytic reactions; VII. Assignment of absolute configurations; VIII. 1H NMR and 13C NMR spectra of compounds; and IX. References.
Rights and permissions
About this article
Cite this article
Zetzsche, L.E., Yazarians, J.A., Chakrabarty, S. et al. Biocatalytic oxidative cross-coupling reactions for biaryl bond formation. Nature 603, 79–85 (2022). https://doi.org/10.1038/s41586-021-04365-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04365-7
This article is cited by
-
Buy one, get one free
Nature Synthesis (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.