Featured
-
-
Article |
 An exercise-inducible metabolite that suppresses feeding and obesity
An exercise-inducible metabolite that suppresses feeding and obesity
A newly identified exercise-induced signalling metabolite—an amidated conjugate of lactate and phenylalanine—can reduce food intake and improve blood glucose homeostasis.
- Veronica L. Li
- , Yang He
- & Jonathan Z. Long
-
Article |
 Structural basis of human separase regulation by securin and CDK1–cyclin B1
Structural basis of human separase regulation by securin and CDK1–cyclin B1
Structures of separase in complex with either securin or cyclin B–CDK1 shed light on the regulation of chromosome separation during the cell cycle.
- Jun Yu
- , Pierre Raia
- & Andreas Boland
-
Article |
 Securin-independent regulation of separase by checkpoint-induced shugoshin–MAD2
Securin-independent regulation of separase by checkpoint-induced shugoshin–MAD2
Shugoshin and MAD2 regulate separase-mediated chromosome separation during mitosis, in parallel to a previously identified mechanism involving the anaphase inhibitor securin.
- Susanne Hellmuth
- , Laura Gómez-H
- & Olaf Stemmann
-
Article |
 Separase-triggered apoptosis enforces minimal length of mitosis
Separase-triggered apoptosis enforces minimal length of mitosis
If early mitosis is too short, separase induces apoptosis by cleaving MCL2 and BCL-XL, thereby eliminating cells that are prone to chromosome missegregation.
- Susanne Hellmuth
- & Olaf Stemmann
-
Article |
 Structural basis of Notch recognition by human γ-secretase
Structural basis of Notch recognition by human γ-secretase
The cryo-electron microscopy structure of human γ-secretase in complex with its substrate Notch reveals pronounced structural rearrangements compared to the apo enzyme, including formation of a β-sheet involving residues from both enzyme and substrate.
- Guanghui Yang
- , Rui Zhou
- & Yigong Shi
-
Article |
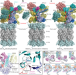 Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome
Cryo-EM structures and dynamics of substrate-engaged human 26S proteasome
Cryo-electron microscopy structures and dynamics of a substrate-engaged human 26S proteasome reveal in atomic detail three principal modes of coordinated ATP hydrolysis that regulate different steps in the degradation of a ubiquitylated protein.
- Yuanchen Dong
- , Shuwen Zhang
- & Youdong Mao
-
Article |
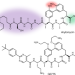 Optimized arylomycins are a new class of Gram-negative antibiotics
Optimized arylomycins are a new class of Gram-negative antibiotics
Chemical optimization of arylomycins results in an inhibitor of bacterial type I signal peptidase that shows activity both against multidrug-resistant clinical isolates of Gram-negative bacteria in vitro and in several in vivo infection models.
- Peter A. Smith
- , Michael F. T. Koehler
- & Christopher E. Heise
-
Letter |
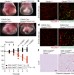 OTULIN limits cell death and inflammation by deubiquitinating LUBAC
OTULIN limits cell death and inflammation by deubiquitinating LUBAC
OTULIN, which removes ubiquitin chains deposited by LUBAC, promotes LUBAC activity by preventing its auto-ubiquitination, thereby supporting normal mouse embryo development and preventing pro-inflammatory cell death in adult mice.
- Klaus Heger
- , Katherine E. Wickliffe
- & Vishva M. Dixit
-
Letter |
 Molecular mechanism for the regulation of yeast separase by securin
Molecular mechanism for the regulation of yeast separase by securin
The crystal structure of yeast separase in complex with its inhibitor securin sheds light on the mechanism of inhibition, in which securin inhibits separase by inserting a short segment into the active site.
- Shukun Luo
- & Liang Tong
-
Article |
 Arginine phosphorylation marks proteins for degradation by a Clp protease
Arginine phosphorylation marks proteins for degradation by a Clp protease
In Gram-positive bacteria, arginine phosphorylation by the McsB kinase functions as a general post-translational marker for Clp-mediated proteolysis.
- Débora Broch Trentini
- , Marcin Józef Suskiewicz
- & Tim Clausen
-
Letter |
 USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites
USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites
The proteasome-associated enzyme USP14 regulates protein degradation by removing ubiquitin from proteins; here it is shown that USP14 removes ubiquitin chains from in vitro generated cyclin B conjugates en bloc and within milliseconds, before the proteasome has a chance to initiate degradation, and proceeds until a single chain remains.
- Byung-Hoon Lee
- , Ying Lu
- & Daniel Finley
-
Letter |
 Structural basis of cohesin cleavage by separase
Structural basis of cohesin cleavage by separase
The crystal structures of the protease domain of separase are reported, showing how separase recognizes cohesin, and how phosphorylation of the cleavage site enhances separase activity.
- Zhonghui Lin
- , Xuelian Luo
- & Hongtao Yu
-
Letter |
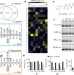 Structure- and function-based design of Plasmodium-selective proteasome inhibitors
Structure- and function-based design of Plasmodium-selective proteasome inhibitors
Structural and functional characterizations show that the specificity of the Plasmodium falciparum proteasome is sufficiently unique from that of the human proteasome to allow selective targeting with inhibitors.
- Hao Li
- , Anthony J. O’Donoghue
- & Matthew Bogyo
-
Article |
 An atomic structure of human γ-secretase
An atomic structure of human γ-secretase
The atomic structure of human γ-secretase at 3.4 Å resolution, determined by single-particle cryo-electron microscopy.
- Xiao-chen Bai
- , Chuangye Yan
- & Yigong Shi
-
Letter |
 Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating
Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating
Calcium potently stimulates proteolysis by endogenous rhomboid-4, an intramembrane protease that contains a cytoplasmic calcium-binding EF-hand domain.
- Rosanna P. Baker
- & Siniša Urban
-
Article |
 Three-dimensional structure of human γ-secretase
Three-dimensional structure of human γ-secretase
The three-dimensional structure of intact human γ-secretase complex at 4.5 Å resolution is revealed by cryo-electron-microscopy single-particle analysis; the complex comprises a horseshoe-shaped transmembrane domain containing 19 transmembrane segments, and a large extracellular domain from nicastrin, which sits immediately above the hollow space formed by the horseshoe.
- Peilong Lu
- , Xiao-chen Bai
- & Yigong Shi
-
Article |
 Structure of a presenilin family intramembrane aspartate protease
Structure of a presenilin family intramembrane aspartate protease
Presenilin, the catalytic component of γ-secretase, cleaves amyloid precursor protein into short peptides that form the plaques that are found in the brains of patients with Alzheimer’s disease; here the structure of a presenilin homologue is described, which will serve as a framework for understanding the mechanisms of action of presenilin and γ-secretase.
- Xiaochun Li
- , Shangyu Dang
- & Yigong Shi
-
Article |
 Type VI secretion delivers bacteriolytic effectors to target cells
Type VI secretion delivers bacteriolytic effectors to target cells
- Alistair B. Russell
- , Rachel D. Hood
- & Joseph D. Mougous
-
-
News & Views |
 Blocking ubiquitin transfer
Blocking ubiquitin transfer
The protein OTUB1 inhibits DNA repair without using its enzymatic activity. Instead, it sequesters a protein that is required for the assembly of certain forms of ubiquitin chain, which function as key signals during repair.
- April Rose
- & Christian Schlieker
-
Article |
 Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte
Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte
To survive and evade host responses, malaria parasites export several hundred proteins into the host cell on infection. A feature of these proteins is a conserved, pentameric motif that is cleaved by an unknown protease before export. This is one of two independent studies revealing the identity of the protease as plasmepsin V, an aspartic acid protease located in the endoplasmic reticulum. This enzyme is essential for parasite viability and is an attractive candidate for drug development.
- Ilaria Russo
- , Shalon Babbitt
- & Daniel E. Goldberg
-
Article |
 An aspartyl protease directs malaria effector proteins to the host cell
An aspartyl protease directs malaria effector proteins to the host cell
To survive and evade host responses, malaria parasites export several hundred proteins into the host cell on infection. A feature of these proteins is a conserved, pentameric motif that is cleaved by an unknown protease before export. This is one of two independent studies revealing the identity of the protease as plasmepsin V, an aspartic acid protease located in the endoplasmic reticulum. This enzyme is essential for parasite viability and is an attractive candidate for drug development.
- Justin A. Boddey
- , Anthony N. Hodder
- & Alan F. Cowman

 Antiviral signalling by a cyclic nucleotide activated CRISPR protease
Antiviral signalling by a cyclic nucleotide activated CRISPR protease The crystal structure of GXGD membrane protease FlaK
The crystal structure of GXGD membrane protease FlaK