« Prev Next »
Organisms that reproduce sexually are thought to have an advantage over organisms that reproduce asexually, because novel combinations of genes are possible in each generation. Furthermore, with few exceptions, each individual in a population of sexually reproducing organisms has a distinct genetic composition. We have meiosis to thank for this variety.
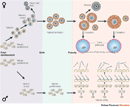
Meiosis, from the Greek word meioun, meaning "to make small," refers to the specialized process by which germ cells divide to produce gametes. Because the chromosome number of a species remains the same from one generation to the next, the chromosome number of germ cells must be reduced by half during meiosis. To accomplish this feat, meiosis, unlike mitosis, involves a single round of DNA replication followed by two rounds of cell division (Figure 1). Meiosis also differs from mitosis in that it involves a process known as recombination, during which chromosomes exchange segments with one another. As a result, the gametes produced during meiosis are genetically unique.
Researchers' initial understanding of meiosis was based upon careful observations of chromosome behavior using light microscopes. Then, in the 1950s, electron microscopy provided scientists with a glimpse of the intricate structures formed when chromosomes recombine. More recently, researchers have been able to identify some of the molecular players in meiosis from biochemical analyses of meiotic chromosomes and from genetic studies of meiosis-specific mutants.
Meiosis Is a Highly Regulated Process
Meiosis represents a survival mechanism for some simple eukaryotes such as yeast. When conditions are favorable, yeast reproduce asexually by mitosis. When nutrients become limited, however, yeast enter meiosis. The commitment to meiosis enhances the probability that the next generation will survive, because genetic recombination during meiosis generates four reproductive spores per cell, each of which has a novel genotype. The entry of yeast into meiosis is a highly regulated process that involves significant changes in gene transcription (Lopez-Maury et al., 2008). By analyzing yeast mutants that are unable to complete the various events of meiosis, investigators have been able to identify some of the molecules involved in this complex process. These controls have been strongly conserved during evolution, so such yeast experiments have provided valuable insight into meiosis in multicellular organisms as well.
In most multicellular organisms, meiosis is restricted to germ cells that are set aside in early development. The germ cells reside in specialized environments provided by the gonads, or sex organs. Within the gonads, the germ cells proliferate by mitosis until they receive the right signals to enter meiosis.
In mammals, the timing of meiosis differs greatly between males and females (Figure 2). Male germ cells, or spermatogonia, do not enter meiosis until after puberty. Even then, only limited numbers of spermatogonia enter meiosis at any one time, such that adult males retain a pool of actively dividing spermatogonia that acts as a stem cell population. On the other hand, meiosis occurs with quite different kinetics in mammalian females. Female germ cells, or oogonia, stop dividing and enter meiosis within the fetal ovary. Those germ cells that enter meiosis become oocytes, the source of future eggs. Consequently, females are born with a finite number of oocytes arrested in the first meiotic prophase. Within the ovary, these oocytes grow within follicle structures containing large numbers of support cells. The oocytes will reenter meiosis only when they are ovulated in response to hormones. Human females, for example, are born with hundreds of thousands of oocytes that remain arrested in the first meiotic prophase for decades. Over time, the quality of the oocytes may deteriorate; indeed, researchers know that many oocytes die and disappear from ovaries in a process known as atresia.
Meiosis Consists of a Reduction Division and an Equational Division
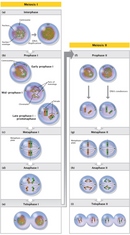
Two divisions, meiosis I and meiosis II, are required to produce gametes (Figure 3). Meiosis I is a unique cell division that occurs only in germ cells; meiosis II is similar to a mitotic division. Before germ cells enter meiosis, they are generally diploid, meaning that they have two homologous copies of each chromosome. Then, just before a germ cell enters meiosis, it duplicates its DNA so that the cell contains four DNA copies distributed between two pairs of homologous chromosomes.
Meiosis I
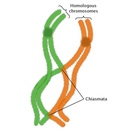
Compared to mitosis, which can take place in a matter of minutes, meiosis is a slow process, largely because of the time that the cell spends in prophase I. During prophase I, the pairs of homologous chromosomes come together to form a tetrad or bivalent, which contains four chromatids. Recombination can occur between any two chromatids within this tetrad structure. (The recombination process is discussed in greater detail later in this article.) Crossovers between homologous chromatids can be visualized in structures known as chiasmata, which appear late in prophase I (Figure 4). Chiasmata are essential for accurate meioses. In fact, cells that fail to form chiasmata may not be able to segregate their chromosomes properly during anaphase, thereby producing aneuploid gametes with abnormal numbers of chromosomes (Hassold & Hunt, 2001).
At the end of prometaphase I, meiotic cells enter metaphase I. Here, in sharp contrast to mitosis, pairs of homologous chromosomes line up opposite each other on the metaphase plate, with the kinetochores on sister chromatids facing the same pole. Pairs of sex chromosomes also align on the metaphase plate. In human males, the Y chromosome pairs and crosses over with the X chromosome. These crossovers are possible because the X and Y chromosomes have small regions of similarity near their tips. Crossover between these homologous regions ensures that the sex chromosomes will segregate properly when the cell divides.
Next, during anaphase I, the pairs of homologous chromosomes separate to different daughter cells. Before the pairs can separate, however, the crossovers between chromosomes must be resolved and meiosis-specific cohesins must be released from the arms of the sister chromatids. Failure to separate the pairs of chromosomes to different daughter cells is referred to as nondisjunction, and it is a major source of aneuploidy. Overall, aneuploidy appears to be a relatively frequent event in humans. In fact, the frequency of aneuploidy in humans has been estimated to be as high as 10% to 30%, and this frequency increases sharply with maternal age (Hassold & Hunt, 2001).
Meiosis II
Following meiosis I, the daughter cells enter meiosis II without passing through interphase or replicating their DNA. Meiosis II resembles a mitotic division, except that the chromosome number has been reduced by half. Thus, the products of meiosis II are four haploid cells that contain a single copy of each chromosome.
In mammals, the number of viable gametes obtained from meiosis differs between males and females. In males, four haploid spermatids of similar size are produced from each spermatogonium. In females, however, the cytoplasmic divisions that occur during meiosis are very asymmetric. Fully grown oocytes within the ovary are already much larger than sperm, and the future egg retains most of this volume as it passes through meiosis. As a consequence, only one functional oocyte is obtained from each female meiosis (Figure 2). The other three haploid cells are pinched off from the oocyte as polar bodies that contain very little cytoplasm.
Recombination Occurs During the Prolonged Prophase of Meiosis I
Prophase I is the longest and arguably most important segment of meiosis, because recombination occurs during this interval. For many years, cytologists have divided prophase I into multiple segments, based upon the appearance of the meiotic chromosomes. Thus, these scientists have described a leptotene (from the Greek for "thin threads") phase, which is followed sequentially by the zygotene (from the Greek for "paired threads"), pachytene (from the Greek for "thick threads"), and diplotene (from the Greek for "two threads") phases. In recent years, cytology and genetics have come together so that researchers now understand some of the molecular events responsible for the stunning rearrangements of chromatin observed during these phases.
Recall that prophase I begins with the alignment of homologous chromosome pairs. Historically, alignment has been a difficult problem to approach experimentally, but new techniques for visualizing individual chromosomes with fluorescent probes are providing insights into the process. Recent experiments suggest that chromosomes from some species have specific sequences that act as pairing centers for alignment. In some cases, alignment appears to begin as early as interphase, when homologous chromosomes occupy the same territory within the interphase nucleus (Figure 5). However, in other species, including yeast and humans, chromosomes do not pair with each other until double-stranded breaks (DSBs) appear in the DNA (Gerton & Hawley, 2005). The formation of DSBs is catalyzed by highly conserved proteins with topoisomerase activity that resemble the Spo11 protein from yeast. Genetic studies have shown that Spo11 activity is essential for meiosis in yeast, because spo11 mutants fail to sporulate.
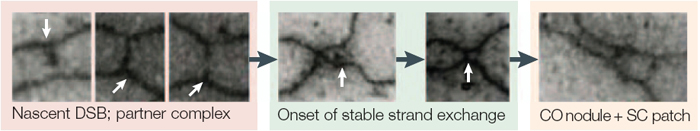
Following the DSBs, one DNA strand is trimmed back, leaving a 3′-overhang that "invades" a homologous sequence on another chromatid. As the invading strand is extended, a remarkable structure called synaptonemal complex (SC) develops around the paired homologues and holds them in close register, or synapsis. The stability of the SC increases as the invading strand first extends into the homologue and then is recaptured by the broken chromatid, forming double Holliday junctions. Investigators have been able to observe the process of SC formation with electron microscopy in meiocytes from the Allium plant (Figure 6). Bridges approximately 400 nanometers long begin to form between the paired homologues following the DSB. Only a fraction of these bridges will mature into SC; moreover, not all Holliday junctions will mature into crossover sites. Recombination will thus occur at only a few sites along each chromosome, and the products of the crossover will become visible as chiasmata in diplotene after the SC has disappeared (Zickler & Kleckner, 1999).
References and Recommended Reading
Gerton, J. L., & Hawley, R. S. Homologous chromosome interactions in meiosis: Diversity amidst conservation. Nature Reviews Genetics 6, 477–487 (2005) doi:10.1038/nrg1614 (link to article)
Hassold, T., & Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nature Reviews Genetics 2, 280–291 (2001) doi:10.1038/35066065 (link to article)
Lopez-Maury, L., Marguerat, S., & Bahler, J. Tuning gene expression to changing environments: From rapid responses to evolutionary adaptation. Nature Reviews Genetics 9, 583–593 (2008) doi:10.1038/nrg2398 (link to article)
Marston, A. L., & Amon, A. Meiosis: Cell-cycle controls shuffle and deal. Nature Reviews Molecular Cell Biology 5, 993–1008 (2004) doi:10.1038/nrm1526 (link to article)
Page, S. L., & Hawley, R. S. Chromosome choreography: The meiotic ballet. Science 301, 785–789 (2003)
Petes, T. D. Meiotic recombination hot spots and cold spots. Nature Reviews Genetics 2, 360–369 (2001) doi:10.1038/35072078 (link to article)
Zickler, D., & Kleckner, N. Meiotic chromosomes: Integrating structure and function. Annual Review of Genetics 33, 603–754 (1999)




 Figure 1
Figure 1