« Prev Next »
Centromeres Are Required for Accurate Segregation of Chromosomes
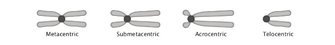
With a few exceptions, eukaryotic chromosomes have a single centromere that ensures their accurate segregation during mitosis. Chromosomes that lack centromeres segregate randomly during mitosis and are eventually lost from cells. At the other extreme, chromosomes with multiple centromeres are subject to fragmentation if the centromeres become attached to opposite spindle poles by way of their kinetochores.
Centromeres Are Regions of Specialized Chromatin
This article focuses on the centromeres of mammalian chromosomes, particularly those in humans. Much of what is known about the composition of these structures is a direct result of the serendipitous discovery in 1985 of antibodies that reacted with human centromeres. Specifically, during that year, researchers William C. Earnshaw and Naomi Rothfield reported that antibodies from the sera of patients with scleroderma, an autoimmune disease, reacted with centromeres. They named the proteins that reacted with these antibodies centromere proteins (CENP) A, B, and C. The antisera from scleroderma patients thus provided investigators with reagents that could be used to identify functionally important proteins in the centromere (Earnshaw & Rothfield, 1985).
Within a few years of this discovery, other scientists began to learn more about the role of each centromere protein. For instance, one of the centromeric proteins, CENP-A, was identified as an unusual variant of histone H3 that replaces H3 in nucleosomes found exclusively at the centromere (Palmer et al., 1991). Thus, the presence of CENP-A can be used to distinguish the comparatively small number of centromere-specific nucleosomes in a chromosome from the great majority of other nucleosomes. The activity of CENP-A appears to be fundamental to centromeres, as cells that lack CENP-A fail to divide properly. In addition, homologues of CENP-A are present in all eukaryotes, indicating that this protein's function has been strongly conserved during evolution.
Meanwhile, subsequent research identified CENP-B as a protein that binds to a simple sequence repeated many times in centromeric DNA. The centromeres of human chromosomes are heterochromatic regions that consist largely of a repeated sequence known as alpha satellite DNA. A single unit of alpha satellite DNA is 171 base pairs in length, each of which contains a 17-base-pair binding site for CENP-B, referred to as the CENP-B box. Because centromeres vary in length, an individual chromosome can contain anywhere from hundreds to thousands of potential CENP-B boxes. Experiments show that CENP-B works as a dimer, so it may be able to cross-link two nearby satellite sequences, thereby contributing to the higher-order structure of the chromosome. Figure 3 shows one model of how CENP-A and CENP-B might interact with alpha satellite DNA. In this model, CENP-B dimers bind to alpha satellite sequences on two different nucleosomes (Kitagawa & Hieter, 2001).
CENP-A and CENP-B are inner centromere proteins that interact directly with DNA. Other CENP proteins play a variety of different roles (Musacchio & Salmon, 2007; Cheeseman & Desai, 2008). Today, the number of known CENP proteins is about 20, and that number may increase as our understanding of the kinetochore grows.
Kinetochores Assemble at Centromeres
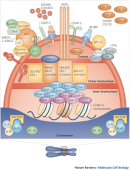
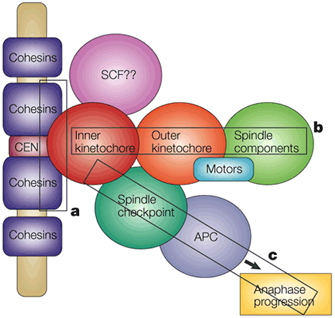
A detailed discussion of the kinetochore is beyond the scope of this article, but Figure 5 shows one model of how proteins might be organized within the kinetochore. Note the many CENP molecules that are located within the inner kinetochore. Clearly, the kinetochore structure is quite complex, and our understanding of kinetochores will continue to grow as new research results are published.
Centromere Identity Is Preserved During Cell Division
Kinetochores are dynamic assemblies of proteins with compositions that change during the cell cycle. With the exception of CENP-A and some other proteins in the inner kinetochore, the structure disassembles at the end of mitosis. CENP-A remains associated with the centromere throughout the cell cycle because it is an integral component of the nucleosome. During the S phase that follows mitotic division, the presence of CENP-A nucleosomes marks the position of the centromere on the two daughter DNA strands. In keeping with the semiconservative nature of chromatin replication, CENP-A-containing nucleosomes are divided more or less equally between the two daughter DNA strands. Following replication, a specific CENP-A loading factor is thought to recruit additional CENP-A to the centromeres (Figure 5; note that the different loading factors shown in purple also recruit H3 to chromatin). By this kind of mechanism, centromeres are able to maintain stable positions on chromosomes through multiple rounds of cell division (Sullivan et al., 2001).
Sister Chromatids Are Joined Together at Centromeres
In addition to their kinetochore-related function, centromeres perform another essential role in mitosis by serving as the sites of sister chromatid cohesion. For accurate mitoses, sister chromatids must remain attached until the spindle checkpoint has been passed. As noted elsewhere, the attachment of sister chromatids is mediated by the cohesin complex of proteins. As Figure 5 shows, chromatid attachment occurs on a different face of the chromosome from the kinetochore. As a cell enters anaphase, cohesin is precipitously degraded, and the cell's sister chromatids separate to opposite poles of the spindle.
Centromeres Still Hold Many Secrets
Considering the many essential roles that centromeres play in mitosis, they are truly at the center of this process. Experiments over the past 30 years have shown that the centromere is far more than simply a "primary constriction" in a chromosome. We now know that centromeres are dynamic assemblies of chromatin and that specialized functional regions exist within each centromere. Given the large amount of current research interest in centromere biology, it seems likely that the centromere has a few more surprises in store for us.
References and Recommended Reading
Cheeseman, I. M., & Desai, A. Molecular architecture of the kinetochore-microtubule interface. Nature Reviews Molecular Cell Biology 9, 33–46 (2008) doi:10.1038/nrm2310 (link to article)
Earnshaw, W. C., & Rothfield, N. Identification of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma 91, 313–321 (1985)
Kitagawa, K., & Hieter, P. Evolutionary conservation between budding yeast and human kinetochores. Nature Reviews Molecular Cell Biology 2, 678–687 (2001) doi:10.1038/35089568 (link to article)
Musacchio, A., & Salmon, E. D. The spindle-assembly checkpoint in space and time. Nature Reviews Molecular Cell Biology 8, 379–393 (2007) doi:10.1038/nrm2163 (link to article)
Palmer, D. K., et al. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proceedings of the National Academy of Sciences 88, 3734–3738 (1991)
Sullivan, B. A., et al. Determining centromere identity: Cyclical stories and forking paths. Nature Reviews Genetics 2, 584–596 (2001) doi:10.1038/35084512 (link to article)



 Figure 1
Figure 1