Featured
-
-
Article |
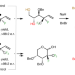 Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Methods to access organofluorine compounds with a trifluoromethyl- and fluoro-substituted carbon stereogenic centre are severely limited. Now, a flexible and stereodivergent approach has been developed for the efficient preparation of homoallylic alcohols containing this moiety. Conversion to polyfluoro furanosides and pyranosides has been demonstrated, which is relevant to antiviral drug development.
- Shibo Xu
- , Juan del Pozo
- & Amir H. Hoveyda
-
News & Views |
 Enriched amino acids
Enriched amino acids
The direct carbon isotope exchange reaction on α-amino acids is highly desirable, as existing labelling methods require several synthetic steps and harsh conditions. Now, an aldehyde-catalysed carboxylate exchange with isotopically labelled *CO2 has enabled the direct formation of 11C, 13C and 14C-labelled α-amino acids.
- Karoline T. Neumann
- & Troels Skrydstrup
-
Article |
 Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2
Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2
Carbon-labelled α-amino acids are valuable compounds in drug development and nuclear medicine, but are difficult and time consuming to prepare. Now, an aldehyde-catalysed method has been developed for the direct C1-labelling of α-amino acids using *CO2 (* = 14, 13, 11), providing access to many proteinogenic and non-natural labelled α-amino acids.
- Odey Bsharat
- , Michael G. J. Doyle
- & Rylan J. Lundgren
-
Article |
 Stereocontrolled acyclic diene metathesis polymerization
Stereocontrolled acyclic diene metathesis polymerization
Polymerization methods that control the cis/trans stereochemistry of repeating alkenes in polyalkenamers remain scarce. Now, an acyclic diene metathesis process has been developed that enables control over the stereochemistry of the polymer backbone. The method harnesses the reactivity of dithiolate Ru carbenes, in combination with cis,cis-diene monomers, to access several classes of polymers with tailored properties.
- Ting-Wei Hsu
- , Samuel J. Kempel
- & Quentin Michaudel
-
Article |
 A soil-inspired dynamically responsive chemical system for microbial modulation
A soil-inspired dynamically responsive chemical system for microbial modulation
Creating hierarchical synthetic materials that can modulate microbial communities remains a great challenge due to the complex interactions between microbiota and their colonized environments. Now, a soil-inspired chemical system that responds to chemical, optical and mechanical stimuli has been developed. The soil-inspired chemical system can enhance microbial cultures and biofuel production, enrich gut bacterial diversity and alleviate ulcerative colitis symptoms.
- Yiliang Lin
- , Xiang Gao
- & Bozhi Tian
-
Article
| Open Access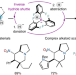 Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks
Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks
The rapid assembly of complex scaffolds in a single step from simple precursors would be an ideal reaction in terms of efficiency and sustainability. Now, the single-step construction of alkaloid-like frameworks from three dynamically assembled precursors has been reported. Using a dual-catalytic system, the transformation involves a hydride shuttle process initiated by a hydride donation event.
- Immo Klose
- , Giovanni Di Mauro
- & Nuno Maulide
-
Article |
 Bending a photonic wire into a ring
Bending a photonic wire into a ring
Meso–meso linked porphyrin arrays have been described as rod-like photonic wires. Now it has been shown that they can be bent into rings using template-directed synthesis. These rings of porphyrins mimic the light-harvesting arrays of chlorophyll molecules responsible for photosynthesis.
- Henrik Gotfredsen
- , Jie-Ren Deng
- & Harry L. Anderson
-
Article |
 An autonomous portable platform for universal chemical synthesis
An autonomous portable platform for universal chemical synthesis
Automated systems, nowadays more commonly used in laboratory settings, are typically fixed to a narrow set of reactions and used within a complex laboratory environment. Now, a portable platform has been developed for the on-demand and on-site multistep synthesis of organic molecules, oligonucleotides and oligopeptides mapped into reactionware systems.
- J. Sebastián Manzano
- , Wenduan Hou
- & Leroy Cronin
-
Article |
 Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Tigilanol tiglate is a therapeutic lead for the treatment of a broad range of cancers. Now, it has been shown that tigilanol tiglate can be synthesized in a time and step economical fashion from phorbol—its naturally abundant biosynthetic precursor. This synthesis provides rapid access to analogues with unprecedented protein kinase C binding activity.
- Paul A. Wender
- , Zachary O. Gentry
- & Edward Njoo
-
Article |
 Total synthesis of structurally diverse pleuromutilin antibiotics
Total synthesis of structurally diverse pleuromutilin antibiotics
General synthetic methods to access pleuromutilin antibiotics are limited due to their complex carbocyclic skeleton. Now, a synthetic platform has been developed to access structurally diverse pleuromutilins with variations at the quaternary C12 position and hydrindanone cores. Seventeen structurally distinct derivatives were prepared and evaluated against a panel of Gram-positive and -negative bacteria.
- Olivia Goethe
- , Mikaela DiBello
- & Seth B. Herzon
-
Article |
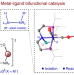 Structure, reactivity and catalytic properties of manganese-hydride amidate complexes
Structure, reactivity and catalytic properties of manganese-hydride amidate complexes
Noyori-type hydrogenation catalysts consist of an N–H moiety coordinated to a metal centre. Now, a metal-hydride amidate complex (HMn–NLi) has been isolated and found to have superior reactivity and catalytic performance compared with the corresponding HMn–NH complex, highlighting the superiority of M/NM′ bifunctional catalysis over the classic M/NH bifunctional catalysis for hydrogenation reactions.
- Yujie Wang
- , Shihan Liu
- & Qiang Liu
-
News & Views |
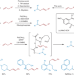 Back to basics
Back to basics
Amines with free N–H groups have long posed a tremendous challenge in transition metal-catalysed amination reactions. Now, use of a bidentate phosphorus ligand enables the palladium-catalysed oxidative amination of simple olefins with Lewis basic amines, with no prefunctionalization, forming both alkyl and aryl allylamines.
- Logan E. Vine
- & Jennifer M. Schomaker
-
Article |
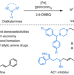 Palladium-catalysed selective oxidative amination of olefins with Lewis basic amines
Palladium-catalysed selective oxidative amination of olefins with Lewis basic amines
Given the importance of amine compounds, methods for their synthesis continue to be in high demand. Now, a palladium-catalysed strategy has been developed for the selective oxidative amination of unactivated olefins with Lewis basic amines, via C(sp3)–H activation, forming architecturally versatile and functionally diverse allylamines in a single step.
- Yangbin Jin
- , Yaru Jing
- & Huanfeng Jiang
-
News & Views |
 The chemistry of errors
The chemistry of errors
The application of machine learning to big data, to make quantitative predictions about reaction outcomes, has been fraught with failure. This is because so many chemical-reaction data are not fit for purpose, but predictions would be less error-prone if synthetic chemists changed their reaction design and reporting practices.
- Jacqueline M. Cole
-
Article |
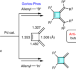 Palladium-catalysed construction of butafulvenes
Palladium-catalysed construction of butafulvenes
Butafulvene is a constitutional isomer of benzene, comprising a cyclobutene skeleton bearing two exocyclic conjugated methylene units. Strategies for the synthesis of butafulvene compounds are currently limited due to its intrinsic high strain energy and anti-aromaticity. Now, palladium-catalysed couplings have been developed for the rapid assembly of symmetric and non-symmetric anti-aromatic butafulvene derivatives.
- Xin Huang
- , Bing-Zhi Chen
- & Shengming Ma
-
Article |
 Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes
Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes
The majority of methods to prepare β-amino acid derivatives require metal-mediated multistep manipulations of pre-functionalized substrates. Now, a metal-free, energy-transfer enabled, highly regioselective aminocarboxylation reaction has been developed, for the single-step installation of both amine and ester functionalities into alkenes or (hetero)arenes. An oxime oxalate ester is used as a bifunctional reagent, supplying C-centred ester and N-centred iminyl radicals.
- Guangying Tan
- , Mowpriya Das
- & Frank Glorius
-
Article |
 Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways
Prebiotic synthesis of α-amino acids and orotate from α-ketoacids potentiates transition to extant metabolic pathways
The apparent disconnect between prebiotic aldehyde-based Strecker synthesis of amino acids and the α-ketoacid-based metabolism of extant biochemistry necessitates an evolutionary switch between these disparate chemistries. Now it has been shown that Bucherer–Bergs reactions of α-ketoacids produce α-amino acids and dihydroorotate, paralleling the biochemical synthesis of orotate and suggesting a more congruent evolutionary pathway from cyanide-based chemistries.
- Sunil Pulletikurti
- , Mahipal Yadav
- & Ramanarayanan Krishnamurthy
-
News & Views |
 Radical ring formation
Radical ring formation
N-heterocycles are valuable motifs in pharmaceuticals and materials, but divergent synthetic strategies are lacking. Now, bifunctional sulfilimines have been shown to form nitrogen-centred radicals under photocatalytic conditions, and subsequent coupling with olefins enables the synthesis of diverse N-heterocycles.
- Prabagar Baskaran
- & Wei Li
-
Article
| Open Access Bifunctional sulfilimines enable synthesis of multiple N-heterocycles from alkenes
Bifunctional sulfilimines enable synthesis of multiple N-heterocycles from alkenes
Intermolecular cyclization reactions using nitrogen-containing building blocks are scarce. Now, bifunctional sulfilimines have been shown to enable the modular construction of a diverse range of N-heterocycles by reacting with alkenes in a single photocatalysed step. Both sulfilimines and alkenes are easily accessible, providing access to a wide range of N-heterocycles with different ring types, ring sizes and substituents on the skeleton.
- Qiang Cheng
- , Zibo Bai
- & Tobias Ritter
-
Article |
 Chemoenzymatic synthesis of fluorinated polyketides
Chemoenzymatic synthesis of fluorinated polyketides
The introduction of fluorine into a drug molecule can alter the biological responses to it, including modulating bioavailability, pharmacokinetics and selectivity. Now, a hybrid polyketide/fatty acid synthase multienzyme has been designed to incorporate fluorinated precursors during polyketide biosynthesis in an approach that provides new chemoenzymatic access to fluorinated natural compounds.
- Alexander Rittner
- , Mirko Joppe
- & Martin Grininger
-
News & Views |
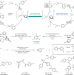 N2O revalorization
N2O revalorization
Nitrous oxide is traditionally considered to be an inert molecule, and methods for its activation and utilization are currently limited. Now, a strategy has been developed — involving an organometallic Baeyer–Villiger step — for the conversion of aryl halides to phenols under mild conditions, using N2O as the oxygen source.
- Jun-Jie Chen
- & Huan-Ming Huang
-
Article |
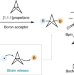 Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane
Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane
Methods to access bicyclo[1.1.1]pentane building blocks are limited, with current routes requiring multiple steps. Now, a diverse array of bicyclo[1.1.1]pentane boronates can be accessed via a multi-component reaction in a single step. Alkyl, aryl and alkenyl substructures can be installed onto bicyclo[1.1.1]pentane boronates by the use of carboxylic acids and organohalides.
- Weizhe Dong
- , Expédite Yen-Pon
- & Gary A. Molander
-
Article |
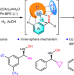 Nickel-catalysed asymmetric hydrogenation of oximes
Nickel-catalysed asymmetric hydrogenation of oximes
The asymmetric hydrogenation of oximes to chiral hydroxylamines is a long-standing challenge because of the labile N–O bond and inert C=N bond. Now, it has been shown that this reaction can be catalysed with a chiral nickel complex, and the weak interactions between catalyst and substrate are found to play a crucial role.
- Bowen Li
- , Jianzhong Chen
- & Wanbin Zhang
-
Article |
 Taming phosphorus mononitride
Taming phosphorus mononitride
Precursors for the release of phosphorus mononitride in solution under mild conditions have remained elusive. Now, an explosive anthracene-stabilized azidophosphine has shown PN transfer reactivity in the synthesis of an Fe–NP complex. The PN ligand is N-bonded, as the Fe–N interaction shows significant covalent character and a less unfavourable Pauli repulsion than its Fe–P counterpart.
- André K. Eckhardt
- , Martin-Louis Y. Riu
- & Christopher C. Cummins
-
Article |
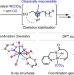 Low-valent tungsten redox catalysis enables controlled isomerization and carbonylative functionalization of alkenes
Low-valent tungsten redox catalysis enables controlled isomerization and carbonylative functionalization of alkenes
The regioselectivity of tandem isomerization/hydrocarbonylation reactions is typically dictated by thermodynamics and there are limitations on the isomerization of internal alkenes. Now, it has been shown that a low-valent-tungsten catalyst controls the isomerization of alkenes to classically challenging unactivated internal positions and, with the aid of a directing group, enables subsequent addition of hydrogen and carbon monoxide.
- Tanner C. Jankins
- , William C. Bell
- & Keary M. Engle
-
Article |
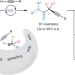 Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
A general copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of diverse racemic tertiary alkyl halides with terminal alkynes has been developed, forging all-carbon quaternary stereocentres. Key to the success is the rational design of chiral anionic N,N,N-ligands tailor-made for the computationally predicted outer-sphere radical group transfer pathway.
- Fu-Li Wang
- , Chang-Jiang Yang
- & Xin-Yuan Liu
-
Article |
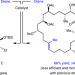 E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing
E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing
Many bioactive compounds are trisubstituted macrocyclic alkenes, but use of current methods often results in poor yields and low stereoselectivity. Now, a ring-closing metathesis strategy has been developed that enables these compounds to be prepared efficiently and in either stereoisomeric form: an approach that may prove useful in the late stages of total syntheses, for skeletal editing and in drug discovery.
- Yucheng Mu
- , Felix W. W. Hartrampf
- & Amir H. Hoveyda
-
Article |
 In situ multiscale probing of the synthesis of a Ni-rich layered oxide cathode reveals reaction heterogeneity driven by competing kinetic pathways
In situ multiscale probing of the synthesis of a Ni-rich layered oxide cathode reveals reaction heterogeneity driven by competing kinetic pathways
Nickel-rich layered oxides, such as NCM622, are promising cathode materials for lithium batteries, but chemo-mechanical failures hinder their practical application. Now the solid-state synthesis of NCM622 has been studied using multiscale in situ techniques, and kinetic competition between precursor decomposition and lithiation has been observed to lead to spatially heterogeneous intermediates and the formation of defects that are detrimental to cycling.
- Hyeokjun Park
- , Hayoung Park
- & Kisuk Kang
-
Article |
 Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation
Halogen-bond-assisted radical activation of glycosyl donors enables mild and stereoconvergent 1,2-cis-glycosylation
Most chemical glycosylation methods operate by acid-promoted, ionic activation of donors. Now, by exploiting the formation of a halogen-bond complex, the activation of glycosyl donors was achieved via a visible light-promoted radical cascade process, resulting in a general, simple and mild way to build challenging 1,2-cis-glycosidic bonds.
- Chen Zhang
- , Hao Zuo
- & Dawen Niu
-
News & Views |
 White phosphorus first
White phosphorus first
Many of the methods used to make phosphines proceed via phosphorus trichloride-based intermediates, leading to chloride waste that is difficult to recycle. It has now been shown that this disadvantage can be overcome by using a method that directly converts white phosphorus into value-added phosphorus compounds.
- Hansjörg Grützmacher
-
Article |
 Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
Stereocontrolled 1,3-nitrogen migration to access chiral α-amino acids
A straightforward method for synthesizing optically active α-amino acids from abundant carboxylic acids has been developed. Based on a nitrene-mediated stereocontrolled 1,3-nitrogen shift, this approach provides access to a large variety of unnatural α-amino acids with aryl, allyl, propargyl and alkyl side chains and enables late-stage amination of carboxylic-acid-containing drugs.
- Chen-Xi Ye
- , Xiang Shen
- & Eric Meggers
-
Article |
 Direct conversion of white phosphorus to versatile phosphorus transfer reagents via oxidative onioation
Direct conversion of white phosphorus to versatile phosphorus transfer reagents via oxidative onioation
White phosphorus (P4) is selectively transformed by oxidative onioation into salts of P1-transfer reagents that feature reactive P–N bonds. These P1 compounds can be used in P–N, P–O and P–C bond-forming reactions to form value-added phosphorus chemicals, representing a viable alternative to the most relevant, yet problematic, P(III) precursor, namely PCl3.
- Maximilian Donath
- , Kai Schwedtmann
- & Jan J. Weigand
-
Article |
 Stereodefined alkenes with a fluoro-chloro terminus as a uniquely enabling compound class
Stereodefined alkenes with a fluoro-chloro terminus as a uniquely enabling compound class
Stereochemically defined trisubstituted alkenyl fluorides are important compounds for drug discovery, agrochemical development and materials science, but their preparation is challenging. Now, a practical and widely applicable catalytic strategy enables access to a large assortment of olefins bearing a fluoro-chloro terminus. Subsequent cross-coupling generates the corresponding trisubstituted alkenyl fluorides.
- Qinghe Liu
- , Yucheng Mu
- & Amir H. Hoveyda
-
Article |
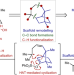 Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy
Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy
Generating strategies that use modern synthetic methods to access privileged chemical space is a central goal of total synthesis. Now, a strategy that is orthogonal to classic approaches, coupled with C–H functionalization methods, leads to the shortest synthesis of the sesquiterpenoid longiborneol and divergent syntheses of oxygenated longiborneol congeners.
- Robert F. Lusi
- , Goh Sennari
- & Richmond Sarpong
-
Article |
 Self-assembly of polyoxometalate clusters into two-dimensional clusterphene structures featuring hexagonal pores
Self-assembly of polyoxometalate clusters into two-dimensional clusterphene structures featuring hexagonal pores
Polyoxometalate clusters have been assembled into two-dimensional ‘clusterphene’ layers that are held together by coordination to lanthanide ions and electrostatic interactions with quaternary ammonium cations. The resulting materials resemble graphene sheets on account of their uniform hexagonal pores and are shown to catalyse epoxidation reactions due to their in-plane electron delocalization.
- Qingda Liu
- , Qinghua Zhang
- & Xun Wang
-
Article |
 Catalytic remote hydrohalogenation of internal alkenes
Catalytic remote hydrohalogenation of internal alkenes
The hydrohalogenation of alkenes generally forms branched alkyl halides. Now, a palladium-catalysed method has been developed for the remote hydrohalogenation of internal and terminal alkenes, enabling the efficient synthesis of linear alkyl halides. The method uses an engineered Pyox ligand with a hydroxy group, which is essential for accelerating the oxidative halogenation.
- Xiang Li
- , Jianbo Jin
- & Guosheng Liu
-
Article |
 Micrometre-scale single-crystalline borophene on a square-lattice Cu(100) surface
Micrometre-scale single-crystalline borophene on a square-lattice Cu(100) surface
Borophene, a two-dimensional boron sheet, can adopt a variety of polymorphic structures that are predicted to possess interesting and potentially useful electronic properties. Micrometre-scale single-crystal borophene domains have now been grown on a square-lattice Cu(100) surface. The resulting boron sheets feature a rectangular unit cell, intrinsic stripe modulations and an unusual electron band structure.
- Rongting Wu
- , Stephen Eltinge
- & Ivan Božović
-
News & Views |
 Simple strategy towards amide bioisosteres
Simple strategy towards amide bioisosteres
Aryl aminooxetanes are used as amide bioisosteres in drug discovery but there are limited strategies for synthesizing them. Now, an approach has been developed that simplifies the synthesis of these privileged motifs, enabling a broad range of amines to be used.
- Malcolm P. Huestis
- & Jack A. Terrett
-
Article |
 Amino-oxetanes as amide isosteres by an alternative defluorosulfonylative coupling of sulfonyl fluorides
Amino-oxetanes as amide isosteres by an alternative defluorosulfonylative coupling of sulfonyl fluorides
Sulfonyl fluorides typically react with nucleophiles exclusively at sulfur, leading to the substitution of fluoride, as is the case in SuFEx reactions. Now, an alternative defluorosulfonylative reaction has been developed, coupling 3-aryloxetane sulfonyl fluorides with amines to generate amino-oxetanes. The mild conditions and high functional group tolerance enable the preparation of oxetane analogues of benzamide drugs via oxetane carbocation intermediates.
- Juan J. Rojas
- , Rosemary A. Croft
- & James A. Bull
-
Article
| Open Access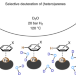 Scalable and selective deuteration of (hetero)arenes
Scalable and selective deuteration of (hetero)arenes
A method for the selective deuteration of anilines, indoles, phenols and heterocyclic compounds, including natural products and other bioactive molecules, has been developed. The nanostructured iron catalyst that underpins this process is prepared by combining cellulose with iron salts and has been used for the preparation of deuterated compounds on up to a kilogram scale.
- Wu Li
- , Jabor Rabeah
- & Matthias Beller
-
Article |
 Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu(ii) carboxylates
Decarboxylative cross-nucleophile coupling via ligand-to-metal charge transfer photoexcitation of Cu(ii) carboxylates
Direct coupling methods, which do not require substrate prefunctionalization, are highly desirable for the construction of complex molecular scaffolds. Now, a photochemical method has been developed for the direct decarboxylative coupling of carboxylic acids with diverse nitrogen, oxygen and carbon nucleophiles, taking advantage of the photochemistry of copper(II) carboxylate complexes assembled in situ.
- Qi Yukki Li
- , Samuel N. Gockel
- & Tehshik P. Yoon
-
News & Views |
 Defogging the view through a milling jar
Defogging the view through a milling jar
Innovations in instrumentation together with new strategies of data collection and processing have been shown to solve the problem of data quality for time-resolved in situ X-ray diffraction studies on ball milling, opening new horizons in mechanochemistry.
- Elena Boldyreva
-
Article
| Open Access Deaminative chlorination of aminoheterocycles
Deaminative chlorination of aminoheterocycles
Strategies for the selective modification of heteroatom-containing aromatic compounds are in high demand. Using a simple pyrylium reagent and a cheap chloride source, a method has now been developed that can selectively replace NH2 groups attached to heteroaromatic rings with a chlorine atom to give heteroaryl chlorides.
- Clément Ghiazza
- , Teresa Faber
- & Josep Cornella
-
Article |
 Arene radiofluorination enabled by photoredox-mediated halide interconversion
Arene radiofluorination enabled by photoredox-mediated halide interconversion
A photoredox-mediated SNAr reaction has now been developed for the direct radiofluorination of unactivated aryl halides. A series of arenes can be radiofluorinated in a site-selective manner from readily available aryl halide precursors under mild conditions. This strategy allows efficient 19F/18F isotopic exchange, enabling rapid PET probe diversification and clinical tracer preparation.
- Wei Chen
- , Hui Wang
- & Zibo Li
-
Article |
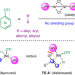 Directing-group-free catalytic dicarbofunctionalization of unactivated alkenes
Directing-group-free catalytic dicarbofunctionalization of unactivated alkenes
Without directing auxiliaries, the addition of carbogenic groups to unactivated alkenes is typically inefficient and suffers from poor regioselectivity. Now, a directing-group-free, nickel catalyst-controlled strategy has been developed, enabling the site-selective dicarbofunctionalization of a broad array of activated and unactivated alkenes.
- Hongyu Wang
- , Chen-Fei Liu
- & Ming Joo Koh
-
Article |
 A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes
A chiral interlocking auxiliary strategy for the synthesis of mechanically planar chiral rotaxanes
The synthesis of chiral interlocked molecules in which the mechanical bond provides the only source of stereochemistry remains challenging. Now, a chiral interlocked auxiliary approach to mechanically planar chiral rotaxanes has been developed and its potential demonstrated through the synthesis of a range of difficult targets with high enantioselectivity.
- Alberto de Juan
- , David Lozano
- & Stephen M. Goldup
-
Article |
 Visible light-driven conjunctive olefination
Visible light-driven conjunctive olefination
A conjunctive olefination between aldehydes and carboxylic acids has been developed by merging photoredox catalysis with the Wittig reaction. The process uses a readily available phosphonium salt to join together complex molecular fragments with high functional group tolerance and minimal use of protecting groups, enabling access to coupling products with user-defined geometries.
- Dario Filippini
- & Mattia Silvi
-
News & Views |
 More details on diazoolefins
More details on diazoolefins
Diazoolefins are long-sought-after compounds, but experimental evidence for them is limited because of their high reactivity. Now, it has been shown that the reaction of N-heterocyclic olefins with nitrous oxide provides access to diazoolefins, enabling structural characterization and applications in organometallic and synthetic chemistry.
- Claire Empel
- & Rene M. Koenigs
-
News & Views |
 Bringing amines back into aziridination
Bringing amines back into aziridination
Aziridines — three-membered nitrogen-containing heterocycles — are important synthetic targets, but N-alkyl aziridines are difficult to synthesize. Now, an electrochemical method, involving a dicationic intermediate, enables the coupling of alkenes and amines.
- Tiffany Piou
- & Louis-Charles Campeau
Browse broader subjects
Browse narrower subjects
- Asymmetric synthesis
- Automation
- Biomimetic synthesis
- Catalyst synthesis
- Chemical libraries
- Diversity-oriented synthesis
- Flow chemistry
- Fluorescent labelling
- Synthetic chemistry methodology
- Microwave chemistry
- Nanoparticle synthesis
- Natural product synthesis
- Polymer synthesis
- Reactive precursors
- Solid-phase synthesis

 Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A