News & Views |
Featured
-
-
Article
| Open Access Accessing elusive σ-type cyclopropenium cation equivalents through redox gold catalysis
Accessing elusive σ-type cyclopropenium cation equivalents through redox gold catalysis
The σ-type cyclopropenium cations (CPCs) are unstable species and currently underdeveloped. Now, an iodine(III)-based cyclopropenyl transfer reagent has been developed, which can generate electrophilic cyclopropenyl-gold(III) species as equivalents of σ-type CPCs. The synthetic potential has been demonstrated by the transfer of σ-type CPCs to terminal alkynes and vinylboronic acids.
- Xiangdong Li
- , Matthew D. Wodrich
- & Jérôme Waser
-
Article |
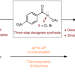 Discovery of N–X anomeric amides as electrophilic halogenation reagents
Discovery of N–X anomeric amides as electrophilic halogenation reagents
Electrophilic halogenation approaches often suffer from low reactivity and chemoselectivity when it comes to complex compounds. Now a class of halogenating reagents based on anomeric amides that can halogenate complex bioactive molecules with diverse functional groups and heterocycles has been developed. The higher reactivity of these anomeric amide reagents is attributed to the energy stored in the pyramidalized nitrogen.
- Yu Wang
- , Cheng Bi
- & Phil S. Baran
-
Article
| Open Access Enabling nucleophilic reactivity in molecular calcium fluoride complexes
Enabling nucleophilic reactivity in molecular calcium fluoride complexes
Calcium difluoride is a source of fluorochemicals, but the reactivity of Ca–F moieties is not well understood. Now a library of molecular Ca–F complexes featuring unique structural motifs has been synthesized, including via fluorochemical defluorination. Studies of mono- and dinuclear systems provided structure–activity relationships for E–F bond formation.
- Job J. C. Struijs
- , Mathias A. Ellwanger
- & Simon Aldridge
-
Article |
 Enantioselective construction of stereogenic-at-sulfur(IV) centres via catalytic acyl transfer sulfinylation
Enantioselective construction of stereogenic-at-sulfur(IV) centres via catalytic acyl transfer sulfinylation
Chiral sulfur pharmacophores are crucial in drug discovery, but the controlled synthesis of sulfinamides with stereogenic-at-sulfur(IV) centres is a long-standing challenge. Now a method for the catalytic asymmetric synthesis of sulfinamides and sulfinate esters has been developed that features the acyl transfer sulfinylation of diverse nucleophiles, including aromatic amines and alcohols, using chiral 4-arylpyridine N-oxides as catalysts.
- Tao Wei
- , Han-Le Wang
- & Hai-Ming Guo
-
Article |
 Persistent organonickel complexes as general platforms for Csp2–Csp3 coupling reactions
Persistent organonickel complexes as general platforms for Csp2–Csp3 coupling reactions
A robust organometallic platform is developed for stoichiometric cross-couplings of common aryl and alkyl electrophiles under a single set of conditions. Inexpensive and persistent organonickel complexes are prepared by electrolysis and implemented in the diversification of drug-like molecules with high reliability from a diverse set of alkyl precursors.
- Long P. Dinh
- , Hunter F. Starbuck
- & Christo S. Sevov
-
Article |
 Proton-triggered topological transformation in superbase-mediated selective polymerization enables access to ultrahigh-molar-mass cyclic polymers
Proton-triggered topological transformation in superbase-mediated selective polymerization enables access to ultrahigh-molar-mass cyclic polymers
The selective synthesis of ultrahigh-molar-mass (UHMM) cyclic polymers from direct polymerization is elusive. Using a chemically recyclable polythioester as a model, it has now been shown that a common superbase mediates living linear-chain growth, followed by proton-triggered linear-to-cyclic topological transformation, producing UHMM cyclic polymers with a narrow dispersity.
- Li Zhou
- , Liam T. Reilly
- & Eugene Y.-X. Chen
-
Article |
 A para- to meta-isomerization of phenols
A para- to meta-isomerization of phenols
Phenols and their derivatives are ubiquitous in nature and important within the chemical industry. Their properties are linked to their substitution patterns, but meta-isomers are underrepresented due to the difficulty of their synthesis. Now we address this challenge by describing a 1,2-transposition of phenols that enables a formal para- to meta-isomerization.
- Simon Edelmann
- & Jean-Philip Lumb
-
Article
| Open Access Stereoretentive enantioconvergent reactions
Stereoretentive enantioconvergent reactions
Enantioconvergent reactions convert both enantiomers of a racemic starting material into a single enantioenriched product. All currently known enantioconvergent processes necessitate the loss or partial loss of the racemic substrate’s stereochemical information. Now, an alternative approach has been developed that proceeds with full retention of the racemic substrate’s configuration.
- Steven H. Bennett
- , Jacob S. Bestwick
- & Andrew L. Lawrence
-
Article |
 Stereodivergent photobiocatalytic radical cyclization through the repurposing and directed evolution of fatty acid photodecarboxylases
Stereodivergent photobiocatalytic radical cyclization through the repurposing and directed evolution of fatty acid photodecarboxylases
Despite their intriguing photochemical activities, natural photoenzymes have not yet been repurposed for new-to-nature activities. Now, by leveraging the strongly oxidizing excited-state flavoquinone cofactor, fatty acid photodecarboxylases were engineered to catalyse unnatural decarboxylative radical cyclization with excellent chemo-, enantio- and diastereoselectivities.
- Shuyun Ju
- , Dian Li
- & Yang Yang
-
Article |
 Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres
Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres
Chiral 1,2-benzazaborines are promising isosteres of naphthalene, but rarely explored due to the lack of efficient synthetic methods. Now, the copper-catalysed enantioselective hydroboration of alkenes with 1,2-benzazaborines has been developed, providing a general platform for the atom-economic and efficient construction of diverse chiral 1,2-benzazaborine compounds bearing a 2-carbon-stereogenic centre or allene skeleton.
- Wanlan Su
- , Jide Zhu
- & Qiuling Song
-
Article
| Open Access An air- and moisture-stable ruthenium precatalyst for diverse reactivity
An air- and moisture-stable ruthenium precatalyst for diverse reactivity
Despite the widespread utility of ruthenium catalysts, many protocols for their use require high temperatures or light irradiation. Now, the synthesis of an air- and moisture-stable ruthenium precatalyst has been reported. This versatile catalyst drives an array of transformations and enables rapid screening and optimization of reactions, revealing previously unknown in situ generated ruthenium complexes.
- Gillian McArthur
- , Jamie H. Docherty
- & Igor Larrosa
-
Article
| Open Access A directed enolization strategy enables by-product-free construction of contiguous stereocentres en route to complex amino acids
A directed enolization strategy enables by-product-free construction of contiguous stereocentres en route to complex amino acids
α-Amino acids possessing β-stereocentres are difficult to synthesize. Now, an iridium-catalysed protocol allows the direct upconversion of simple alkenes and glycine derivatives to give β-substituted α-amino acids with exceptional levels of regio- and stereocontrol. The reaction design is based on exploiting the native directing ability of a glycine-derived N–H unit to facilitate enolization of the adjacent carbonyl.
- Fenglin Hong
- , Timothy P. Aldhous
- & John F. Bower
-
Article |
 Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer
Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer
The redox properties of visible-light-absorbing photosensitizers are limited by the energy of visible photons, but methods using sensitization-initiated electron transfer have recently been developed to address these challenges. Now a multiphoton dual-catalyst strategy has been used to enable the enantioselective de Mayo reaction for the synthesis of enantioenriched 1,5-diketones.
- Xin Sun
- , Yilin Liu
- & Zhiyong Jiang
-
Perspective |
 Bridging the information gap in organic chemical reactions
Bridging the information gap in organic chemical reactions
Lack of standardization, transparency and interaction creates information gaps in scientific publications. Through strategies such as voluntary information management, standardization of reaction set-ups, and smart screening approaches, this Perspective gives guidelines on how to improve data management in publications reporting chemical reactions, focusing on reproducibility, standardization and evaluation of synthetic transformations.
- Malte L. Schrader
- , Felix R. Schäfer
- & Frank Glorius
-
News & Views |
 Copper catalysed asymmetric amination
Copper catalysed asymmetric amination
Chiral amines possessing a stereogenic carbon atom bearing three carbon substituents and one nitrogen substituent are challenging structural motifs to prepare enantioselectively. Now, such motifs have been accessed in high enantiopurities by asymmetric Cu-catalysed propargylic amination using sterically confined ligands.
- Joshua D. Sieber
-
Article |
 Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioenriched α-disubstituted α-ethynylamines are valuable synthons to chiral α-tertiary amines and azacycles, but their facile access remains challenging. Now, sterically confined pyridinebisoxazoline ligands have been developed to facilitate highly enantioselective Cu(I)-catalysed propargylic amination of both aliphatic and aryl ketone-derived propargylic carbonates to give α-tertiary ethynylamines. Related tandem sequences are reported to synthesize quaternary azacycles.
- Zheng Zhang
- , Ying Sun
- & Jian Zhou
-
Article
| Open Access Porphyrin-fused graphene nanoribbons
Porphyrin-fused graphene nanoribbons
The insertion of metal atoms and heteroaromatic units provides a way to tune the optical, electronic and magnetic properties of graphene nanoribbons. Now the synthesis of a porphyrin-fused graphene nanoribbon with a narrow bandgap and high charge mobility has been achieved, and this material used to fabricate field-effect and single-electron transistors.
- Qiang Chen
- , Alessandro Lodi
- & Harry L. Anderson
-
Article |
 Tunable molecular editing of indoles with fluoroalkyl carbenes
Tunable molecular editing of indoles with fluoroalkyl carbenes
The rapid generation of molecular complexity from a given molecular scaffold is crucial to drug discovery and development. Now the chemodivergent molecular editing of indoles using fluoroalkyl carbenes has been developed to modularly access four different types of fluorine-containing N-heterocyclic compound with high molecular complexity.
- Shaopeng Liu
- , Yong Yang
- & Xihe Bi
-
Perspective |
 The impact of UV light on synthetic photochemistry and photocatalysis
The impact of UV light on synthetic photochemistry and photocatalysis
Although generally perceived as an old-fashioned and unselective tool to build molecules, the photochemistry community is now re-discovering the power of UV light and is using key mechanistic information to develop new catalytic processes driven by visible light. This Perspective discusses the progress and impact of UV light in organic synthesis.
- Giulio Goti
- , Kavyasree Manal
- & Luca Dell’Amico
-
Research Briefing |
 Carbon isotope exchange for pharmaceutical radiolabelling through metal-catalysed functional group metathesis
Carbon isotope exchange for pharmaceutical radiolabelling through metal-catalysed functional group metathesis
A method for carbon isotope exchange involving a metal-catalysed metathesis reaction of in situ formed acyl chlorides is demonstrated. The platform provides access to 13C- or 14C-enriched carboxylic acids, including natural products and pharmaceuticals, without the need for radioactive gases, using a single carboxylic acid carbon donor.
-
Article |
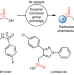 A metal-catalysed functional group metathesis approach to the carbon isotope labelling of carboxylic acids
A metal-catalysed functional group metathesis approach to the carbon isotope labelling of carboxylic acids
The preparation of 14C-labelled compounds is a crucial step in pharmaceutical development but typically requires using toxic, radioactive gases. Now a broadly applicable functional group metathesis reaction has been developed that forms 14C-labelled carboxylic acids in one pot, without added gases, via dynamic exchange with an easily handled carboxylic acid 14C source.
- R. Garrison Kinney
- , José Zgheib
- & Bruce A. Arndtsen
-
News & Views |
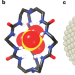 Clever cryptand cage coordinates contaminants
Clever cryptand cage coordinates contaminants
Replicating the ability of enzymes and transport proteins to effectively bind anions is a considerable challenge for supramolecular chemists. A neutral organic cage has now been developed that selectively binds sulfate anions in water.
- Rosemary J. Goodwin
- & Nicholas G. White
-
News & Views |
 Asymmetric construction of sulfur(VI)–fluorine cores
Asymmetric construction of sulfur(VI)–fluorine cores
Molecules containing a chiral S(VI) moiety have found extensive applications in drug design and organic synthesis, despite a lack of diverse asymmetric methods for their creation. Now, a ligand-mediated process has enabled the production of enantioenriched S(VI)–F motifs, providing a foundation for further stereospecific elaborations.
- Patrick R. Melvin
-
Research Briefing |
 Site-selective episulfonium formation on protein surfaces
Site-selective episulfonium formation on protein surfaces
Covalent protein conjugation facilitates the study of biological processes and the synthesis of therapeutic biomacromolecules. A method that uses vinyl thianthrenium reagents for the site-selective formation of highly reactive episulfonium species on proteins is demonstrated. These in situ-formed intermediates react with diverse nucleophiles, providing access to protein conjugates in one step without purification.
-
Article |
 Mapping the mechanisms of oxidative addition in cross-coupling reactions catalysed by phosphine-ligated Ni(0)
Mapping the mechanisms of oxidative addition in cross-coupling reactions catalysed by phosphine-ligated Ni(0)
The mechanism for the oxidative addition of aryl halides to nickel(0)–phosphine complexes was proposed over four decades ago. Now, this elementary reaction, which occurs during common cross-coupling reactions, has been re-examined. Both one- and two-electron pathways occur, and their relative contribution depends on the electronic properties of the reaction partners.
- Christina N. Pierson
- & John F. Hartwig
-
Article |
 Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Chirality is an intrinsic property in unsymmetric three-dimensional molecular assembly, contributing to the utility of the corresponding process and the resulting scaffolds. Now, on the sulfur(VI) hub, a three-step sequential ligand-exchange method has been established with precise stereocontrol, enabling the enantioselective synthesis of optically active S(VI) functional molecules.
- Zhiyuan Peng
- , Shoujun Sun
- & Bing Gao
-
Article
| Open Access Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Achieving selectivity control in allylic arylations is a long-standing challenge in catalysis. Now a rhodium-catalysed system demonstrates chemo-, regio- and enantioselectivity, enabling Suzuki–Miyaura-type arylation with racemic, non-symmetrical, acyclic allylic systems; chelation is speculated to facilitate oxidative addition and enable both enantiomers of the starting material to converge onto a single product.
- Violeta Stojalnikova
- , Stephen J. Webster
- & Stephen P. Fletcher
-
Article
| Open Access The synthesis and characterization of an iron(VII) nitrido complex
The synthesis and characterization of an iron(VII) nitrido complex
Complexes of iron in high oxidation states play a pivotal role as active intermediates in numerous catalytic processes. Now, using a multimethod approach on a single molecular system, it has been shown that a stable high-valent Fe(VI) nitride can be converted to a reactive, superoxidized, heptavalent Fe(VII) nitride.
- Martin Keilwerth
- , Weiqing Mao
- & Karsten Meyer
-
Article |
 Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
While saturated N-heterocycles are widespread motifs in drug discovery, the seven-membered ring azepane is highly underrepresented. Now nitroarenes have been validated as competent substrates for azepane synthesis through conversion into singlet nitrenes for ring enlargement via N insertion and hydrogenolysis. This enables a highly versatile access towards polysubstituted azepanes in just two steps.
- Rory Mykura
- , Raquel Sánchez-Bento
- & Daniele Leonori
-
News & Views |
 Interlocked polyynes towards stable carbynes
Interlocked polyynes towards stable carbynes
Interlocking unstable motifs is a useful way to enhance their stability through shielding protection. Now, stable interlocked polyynes bearing several macrocycles have been prepared, including a [5]rotaxane having 34 contiguous alkynes with properties similar to those of carbyne.
- Adrian Saura-Sanmartin
-
News & Views |
 Sulfur stereochemistry takes centre stage
Sulfur stereochemistry takes centre stage
Directional interactions and three-dimensional functional groups are crucial to medicinal compounds. Consequently, new functional groups require stereocontrolled synthetic methods. Now, an enantiopure building block provides controlled and divergent access to valuable sulfonimidoyl functional groups.
- James A. Bull
-
Article |
 Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent
Asymmetric synthesis of sulfoximines, sulfonimidoyl fluorides and sulfonimidamides enabled by an enantiopure bifunctional S(VI) reagent
The stereochemical control and bifunctional manipulation of chiral sulfur functional groups is a long-standing challenge. Now, an enantiopure bench-stable S(VI) fluoride exchange reagent enables the asymmetric synthesis of sulfoximines, sulfonimidamides and sulfonimidoyl fluorides. The bifunctional nature of this reagent provides a practical method for the introduction of S(VI) functionality.
- Shun Teng
- , Zachary P. Shultz
- & Justin M. Lopchuk
-
Article
| Open Access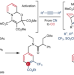 Skeletal editing of pyridines through atom-pair swap from CN to CC
Skeletal editing of pyridines through atom-pair swap from CN to CC
Skeletal editing enables diversification of compounds not possible by applying peripheral editing strategies. Now, a catalyst-free atom-pair swap strategy for pyridine editing has been developed via one-pot sequential dearomatization, cycloaddition and rearomative retrocyclization. Benzenes and naphthalenes with precisely installed functional groups are produced, and the mild conditions enable late-stage skeletal diversification of pyridine cores.
- Qiang Cheng
- , Debkanta Bhattacharya
- & Armido Studer
-
Article
| Open Access Chiral arylsulfinylamides as reagents for visible light-mediated asymmetric alkene aminoarylations
Chiral arylsulfinylamides as reagents for visible light-mediated asymmetric alkene aminoarylations
Single-electron-mediated difunctionalizations of internal olefins allow the simultaneous formation of two contiguous Csp3-stereocentres. Here, we describe enantioenriched arylsulfinylamides as all-in-one reagents for the efficient asymmetric, intermolecular aminoarylation of alkenes. Mechanistic studies revealed an interesting dichotomy in the initiation of the reaction depending on the olefin redox potential.
- Cédric Hervieu
- , Mariia S. Kirillova
- & Cristina Nevado
-
Article |
 A general alkene aminoarylation enabled by N-centred radical reactivity of sulfinamides
A general alkene aminoarylation enabled by N-centred radical reactivity of sulfinamides
Single-step addition of an aryl ring and a protected amine across an alkene is a succinct route to valuable phenethylamine products, but existing methods suffer from limited scope. Now a family of compounds containing a sulfinamide functional group have been developed to react via electrophilic radicals to yield phenethylamines through an aryl migration with precise stereochemical control.
- Efrey A. Noten
- , Cody H. Ng
- & Corey R. J. Stephenson
-
News & Views |
 Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Tetracoordinate boron molecules are the key intermediates in many organoboron-related chemical transformations. Now, using alkynyl tetracoordinate boron species, a nickel-catalysed asymmetric 1,3-metallate shift towards axial chirality has been developed, giving access to various axially chiral alkenes in high efficiency.
- Li-Qing Ren
- & Chuan He
-
Article |
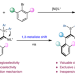 Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Enantioselective transformations based on tetracoordinate borons are elusive. Now, an enantioselective nickel-catalysed strategy for the construction of axially chiral alkenes has been reported, via a 1,3-metallate shift of alkynyl tetracoordinate boron species. The reaction uses readily accessible starting materials and a cheap transition-metal catalyst, and the chemoselectivity, regioselectivity and atroposelectivity could be well controlled.
- Xingxing Ma
- , Mengwei Tan
- & Qiuling Song
-
Article
| Open Access Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation
Chemoselective umpolung of thiols to episulfoniums for cysteine bioconjugation
Cysteine bioconjugation is an important method to modify biomolecules, but synthetic efforts to diversify reactive warheads and the low reactivity of introducible linchpins often impede application in biological laboratories. Now, a thianthrenium-based reagent permits site-selective installation of episulfonium on biomacromolecules, enabling one-step addition of bioorthogonal nucleophiles and further applications in quantitative proteomics and cross-linking.
- Philipp Hartmann
- , Kostiantyn Bohdan
- & Tobias Ritter
-
Article |
 Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Robust protocols for the synthesis of chiral α-tertiary amino acids remain scarce due to the challenge of constructing congested tetrasubstituted stereocentres. Now a cobalt-catalysed enantioselective aza-Barbier reaction of ketimines with various unactivated alkyl halides has been developed, forming diverse chiral α-tertiary amino esters with high enantioselectivity and excellent functional group tolerance.
- Xianqing Wu
- , Hanyu Xia
- & Yifeng Chen
-
Article |
 Organocatalytic aromatization-promoted umpolung reaction of imines
Organocatalytic aromatization-promoted umpolung reaction of imines
The umpolung functionalization of imines bears vast synthetic potential, but polarity inversion is less efficient compared with the carbonyl counterparts. Now, an alternative strategy exploiting chiral phosphoric acid catalytic aromatization has been developed, affording structures possessing a central chirality or a stereogenic C–N axis with high efficiency and enantiocontrol.
- Ye-Hui Chen
- , Meng Duan
- & Bin Tan
-
Article |
 A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution
A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution
Methods for transition-metal-catalysed enantioselective C(sp3)–S bond construction are underdeveloped. Now, by taking advantage of the biomimetic radical homolytic substitution manifold, the copper-catalysed enantioconvergent C(sp3)–S cross-coupling of racemic secondary and tertiary alkyl halides with highly transformable sulfur nucleophiles has been realized. This reaction provides access to an array of α-chiral alkyl organosulfur compounds.
- Yu Tian
- , Xi-Tao Li
- & Xin-Yuan Liu
-
News & Views |
 From feedstock to pharmaceuticals
From feedstock to pharmaceuticals
Fluoroalkyl fragments are ubiquitous motifs in pharmaceuticals and agrochemicals, but their introduction to a given molecule typically involves expensive or difficult-to-handle reagents. Now, the photocatalysed hydrofluoroalkylation of alkenes has been achieved using simple and readily available fluoroalkyl carboxylic acids.
- Fabio Juliá
-
Article
| Open Access Enabling late-stage drug diversification by high-throughput experimentation with geometric deep learning
Enabling late-stage drug diversification by high-throughput experimentation with geometric deep learning
Late-stage functionalization of complex drug molecules is challenging. To address this problem, a discovery platform based on geometric deep learning and high-throughput experimentation was developed. The computational model predicts binary reaction outcome, reaction yield and regioselectivity with low error margins, enabling the functionalization of complex molecules without de novo synthesis.
- David F. Nippa
- , Kenneth Atz
- & Gisbert Schneider
-
Article |
 Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Asymmetric decarboxylation can transform abundant carboxylic acids into valuable chiral molecules but faces major limitations due to the challenging enantiocontrol of proton transfer. Now the use of Brønsted acid catalysis in conjunction with an anchoring group strategy has enabled the decarboxylative protonation of aminomalonic acids to access diverse amino acids.
- Wei-Feng Zheng
- , Jingdan Chen
- & Zhongxing Huang
-
Article
| Open Access Masked alkynes for synthesis of threaded carbon chains
Masked alkynes for synthesis of threaded carbon chains
Long polyynes have fascinating properties but they are difficult to synthesize as a consequence of their high reactivity. Now, it has been shown that cobalt carbonyl complexes can be used as masked alkyne equivalents, enabling the preparation of stable polyyne polyrotaxanes with up to 34 contiguous triple bonds.
- Connor W. Patrick
- , Yueze Gao
- & Harry L. Anderson
-
Article |
 Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids
Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids
Alkene hydrofluoroalkylation offers a promising route to diverse fluoroalkylated compounds but current methods have limitations, such as needing expensive fluoroalkylating reagents. Now, leveraging iron photocatalysis and hydrogen-atom-transfer catalysis, a hydrofluoroalkylation method has been developed that utilizes feedstock chemicals such as trifluoroacetic acid as direct fluoroalkyl radical precursors, providing a redox-neutral, general protocol to introduce fluoroalkyl moieties.
- Kang-Jie Bian
- , Yen-Chu Lu
- & Julian G. West
-
Article |
 Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
The synthesis of optically enriched atropisomers has so far been limited to molecules containing aryl groups. Now a variant of non-aryl atropisomerism has been identified in vinyl sulfoxonium ylides, and an organocatalytic method has been developed to produce these molecules. This type of axial chirality is characterized by restricted rotation of the central C(sp2)–C(sp2) bond.
- Fengjin Wu
- , Yichi Zhang
- & Yong Huang
-
News & Views |
 Enyne difluorination
Enyne difluorination
Fluorination strategies are important in assisting the synthesis of pharmaceuticals. Iodine(I/III) catalysis has become particularly useful for installing gem-difluoro groups but is limited to styrenes. Now, the hypervalent iodane-catalysed difluorination of enynes has enabled access to diverse homopropargylic difluorides.
- Rachel C. Epplin
- & Tanja Gulder
-
Meeting Report |
 Altogether changed, and yet the same
Altogether changed, and yet the same
Organic chemists meet biennially to present exciting developments in the realm of synthesis. Thomas Barber discusses the standout themes of this year’s international synthesis in organic chemistry symposium.
- Thomas Barber

 Cyclopropenium functionalization
Cyclopropenium functionalization