Abstract
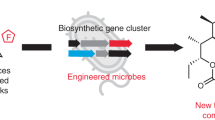
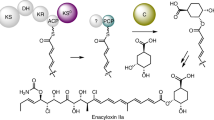
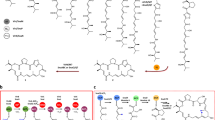
Modification of polyketides with fluorine offers a promising approach to develop new pharmaceuticals. While synthetic chemical methods for site-selective incorporation of fluorine in complex molecules have improved in recent years, approaches for the biosynthetic incorporation of fluorine in natural compounds are still rare. Here, we report a strategy to introduce fluorine into complex polyketides during biosynthesis. We exchanged the native acyltransferase domain of a polyketide synthase, which acts as the gatekeeper for the selection of extender units, with an evolutionarily related but substrate tolerant domain from metazoan type I fatty acid synthase. The resulting polyketide-synthase/fatty-acid-synthase hybrid can utilize fluoromalonyl coenzyme A and fluoromethylmalonyl coenzyme A for polyketide chain extension, introducing fluorine or fluoro-methyl units in polyketide scaffolds. We demonstrate the feasibility of our approach in the chemoenzymatic synthesis of fluorinated 12- and 14-membered macrolactones and fluorinated derivatives of the macrolide antibiotics YC-17 and methymycin.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the main findings of the article, including materials and methods, are described in the Article or Supplementary Information. Alternatively, the data are available from the corresponding author on request.
References
de la Torre, B. G. & Albericio, F. The pharmaceutical industry in 2018. An analysis of FDA drug approvals from the perspective of molecules. Molecules 24, 809–820 (2019).
Newman, D. J. & Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 75, 311–335 (2012).
von Nussbaum, F., Brands, M., Hinzen, B., Weigand, S. & Häbich, D. Antibacterial natural products in medicinal chemistry—exodus or revival? Angew. Chem. Int. Ed. 45, 5072–5129 (2006).
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008).
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Carvalho, M. F. & Oliveira, R. S. Natural production of fluorinated compounds and biotechnological prospects of the fluorinase enzyme. Crit. Rev. Biotechnol. 37, 880–897 (2017).
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Ed. 48, 4688–4716 (2009).
Staunton, J. & Weissman, K. J. Polyketide biosynthesis: a millennium review. Nat. Prod. Rep. 18, 380–416 (2001).
Klaus, M. & Grininger, M. Engineering strategies for rational polyketide synthase design. Nat. Prod. Rep. 35, 1070–1081 (2018).
Kalkreuter, E., CroweTipton, J. M., Lowell, A. N., Sherman, D. H. & Williams, G. J. Engineering the substrate specificity of a modular polyketide synthase for installation of consecutive non-natural extender units. J. Am. Chem. Soc. 141, 1961–1969 (2019).
Walker, M. C. et al. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science 341, 1089–1094 (2013).
Thuronyi, B. W., Privalsky, T. M. & Chang, M. C. Y. Engineered fluorine metabolism and fluoropolymer production in living cells. Angew. Chem. Int. Ed. 56, 13637–13640 (2017).
Rittner, A., Paithankar, K. S., Huu, K. V. & Grininger, M. Characterization of the polyspecific transferase of murine type I fatty acid synthase (FAS) and implications for polyketide synthase (PKS) engineering. ACS Chem. Biol. 13, 723–732 (2018).
Stegemann, F. & Grininger, M. Transacylation kinetics in fatty acid and polyketide synthases and its sensitivity to point mutations. ChemCatChem 13, 2771–2782 (2021).
Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008).
Dutta, S. et al. Structure of a modular polyketide synthase. Nature 510, 512–517 (2014).
Weissman, K. J. The structural biology of biosynthetic megaenzymes. Nat. Chem. Biol. 11, 660–670 (2015).
Wu, N., Kudo, F., Cane, D. E. & Khosla, C. Analysis of the molecular recognition features of individual modules derived from the erythromycin polyketide synthase. J. Am. Chem. Soc. 122, 4847–4852 (2000).
Yoshikuni, Y., Ferrin, T. E. & Keasling, J. D. Designed divergent evolution of enzyme function. Nature 440, 1078–1082 (2006).
Saadi, J. & Wennemers, H. Enantioselective aldol reactions with masked fluoroacetates. Nat. Chem. 8, 276–280 (2016).
Yuzawa, S. et al. Comprehensive in vitro analysis of acyltransferase domain exchanges in modular polyketide synthases and its application for short-chain ketone production. ACS Synth. Biol. 6, 139–147 (2017).
Klaus, M. et al. Solution structure and conformational flexibility of a polyketide synthase module. J. Am. Chem. Soc. Au 1, 2162–2171 (2021).
Shinde, P. B. et al. Combinatorial biosynthesis and antibacterial evaluation of glycosylated derivatives of 12-membered macrolide antibiotic YC-17. J. Biotechnol. 168, 142–148 (2013).
Auerbach, T. et al. Structural basis for the antibacterial activity of the 12-membered-ring mono-sugar macrolide methymycin. Biotechnologia 84, 24–35 (2009).
Almutairi, M. M. et al. Co-produced natural ketolides methymycin and pikromycin inhibit bacterial growth by preventing synthesis of a limited number of proteins. Nucleic Acids Res. 45, 9573–9582 (2017).
Hansen, D. A. et al. Biocatalytic synthesis of pikromycin, methymycin, neomethymycin, novamethymycin, and ketomethymycin. J. Am. Chem. Soc. 135, 11232–11238 (2013).
Hansen, D. A., Koch, A. A. & Sherman, D. H. Identification of a thioesterase bottleneck in the pikromycin pathway through full-module processing of unnatural pentaketides. J. Am. Chem. Soc. 139, 13450–13455 (2017).
Hansen, D. A., Koch, A. A. & Sherman, D. H. Substrate controlled divergence in polyketide synthase catalysis. J. Am. Chem. Soc. 137, 3735–3738 (2015).
Kalkreuter, E. et al. Computationally-guided exchange of substrate selectivity motifs in a modular polyketide synthase acyltransferase. Nat. Commun. 12, 2193 (2021).
Fernandes, P., Martens, E., Bertrand, D. & Pereira, D. The solithromycin journey—it is all in the chemistry. Bioorganic & Medicinal Chemistry 24, 6420–6428 (2016).
Donald, B. J., Surani, S., Deol, H. S., Mbadugha, U. J. & Udeani, G. Spotlight on solithromycin in the treatment of community-acquired bacterial pneumonia: design, development, and potential place in therapy. Drug Dev. Des. Ther. 11, 3559–3566 (2017).
Zhanel, G. G. et al. Solithromycin: a novel fluoroketolide for the treatment of community-acquired bacterial pneumonia. Drugs 76, 1737–1757 (2016).
Poust, S. et al. Divergent mechanistic routes for the formation of gem-dimethyl groups in the biosynthesis of complex polyketides. Angew. Chem. Int. Ed. 54, 2370–2373 (2015).
Koch, A. A. et al. Probing selectivity and creating structural diversity through hybrid polyketide synthases. Angew. Chem. Int. Ed. 59, 13575–13580 (2020).
Jung, W. S. et al. Enhanced heterologous production of desosaminyl macrolides and their hydroxylated derivatives by overexpression of the pikD regulatory gene in Streptomyces venezuelae. Appl. Environ. Microbiol. 74, 1972–1979 (2008).
Tang, Y., Kim, C. Y., Mathews, I. I., Cane, D. E. & Khosla, C. The 2.7-Å crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc. Natl Acad. Sci. USA 103, 11124–11129 (2006).
Ploskoń, E. et al. A mammalian type I fatty acid synthase acyl carrier protein domain does not sequester acyl chains. J. Biol. Chem. 283, 518–528 (2008).
Sharma, K. K. & Boddy, C. N. The thioesterase domain from the pimaricin and erythromycin biosynthetic pathways can catalyze hydrolysis of simple thioester substrates. Bioorg. Med. Chem. Lett. 17, 3034–3037 (2007).
Peter, D. M. et al. Screening and engineering the synthetic potential of carboxylating reductases from central metabolism and polyketide biosynthesis. Angew. Chem. Int. Ed. 54, 13457–13461 (2015).
Dunn, B. J., Watts, K. R., Robbins, T., Cane, D. E. & Khosla, C. Comparative analysis of the substrate specificity of trans- versus cis-acyltransferases of assembly line polyketide synthases. Biochemistry 53, 3796–3806 (2014).
Lowell, A. N. et al. Chemoenzymatic total synthesis and structural diversification of tylactone-based macrolide antibiotics through late-stage polyketide assembly, tailoring, and C—H functionalization. J. Am. Chem. Soc. 139, 7913–7920 (2017).
DeMars, M. D. et al. Biochemical and structural characterization of MycCI, a versatile P450 biocatalyst from the mycinamicin biosynthetic pathway. ACS Chem. Biol. 11, 2642–2654 (2016).
Acknowledgements
This work was supported by a Lichtenberg grant of the Volkswagen Foundation to M.G. (grant number 85701). Further support was received from the LOEWE programme (Landes-Offensive zur Entwicklung wissenschaftlich-ökonomischer Exzellenz) of the state of Hesse conducted within the framework of the MegaSyn Research Cluster to M.G. We thank K. V. Huu and K. Karimi for MS analysis of acyl carrier proteins and K. S. Paithankar for proofreading the manuscript. Further, we are grateful to the Bode group for the extensive support in HPLC-MS and HPLC-HRMS analysis and J. Wirmer-Bartoschek and G. Sentis for support in NMR analysis. D.H.S. is grateful to National Institutes of Health grant R35 GM118101 and the Hans W. Vahlteich Professorship for support.
Author information
Authors and Affiliations
Contributions
A.R. conceived and supervised the project. M.G. and D.H.S. designed the research. A.R. and D.H. performed the expression, purification and mutagenesis of murine KS–MAT constructs. L.M.M. performed global kinetic experiments (with F-Mal-CoA and MM-CoA) and analysed corresponding data under the supervision of A.R.; S.R. performed global kinetic experiments (with F-MM-CoA) and analysed corresponding data under the supervision of M.J.; A.R. and M.J. designed DEBS/FAS hybrids. M.J. performed the expression, purification and analysis of DEBS M6 constructs with respective MS analysis. M.J. and E.H. performed substrate consumption assays by HPLC with ultraviolet spectroscopy. F-Mal-CoA and F-MM-CoA were synthesized by A.R., and the diketide SNAC was synthesized by M.J. Pentaketide and hexaketide substrates were synthesized by J.J.S.; A.R. and M.J. performed semi-synthesis and analysis of compounds 12, (14, 15), 16, 18, 22 and 23 and analysed all data. S.R. performed semi-synthesis of 18 with H1.1 under the supervision of M.J.; J.J.S. performed the biotransformation of compound 18 and analysed data. A.R., M.J., J.J.S., D.H.S. and M.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.R. declares a financial interest as a cofounder of kez.biosolutions GmbH (Potsdam, Germany). All other authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Constance Bailey, Binuraj Menon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24, Tables 1–6, Materials and Methods, notes, spectra and references.
Rights and permissions
About this article
Cite this article
Rittner, A., Joppe, M., Schmidt, J.J. et al. Chemoenzymatic synthesis of fluorinated polyketides. Nat. Chem. 14, 1000–1006 (2022). https://doi.org/10.1038/s41557-022-00996-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00996-z
This article is cited by
-
Structural and mechanistic insights into Quinolone Synthase to address its functional promiscuity
Communications Biology (2024)
-
Biocatalytic enantioselective C(sp3)–H fluorination enabled by directed evolution of non-haem iron enzymes
Nature Synthesis (2024)
-
Enzymology of assembly line synthesis by modular polyketide synthases
Nature Chemical Biology (2023)