Featured
-
-
News & Views |
 Total synthesis of strychnan alkaloids
Total synthesis of strychnan alkaloids
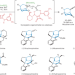
The synthesis of natural products with important biological properties has always fascinated chemists, but the development of rapid, efficient and stereoselective transformations remains challenging. Now, a strategy has been developed to produce several strychnan alkaloids through formation of a common bridged morphan core structure.
- Sylvain Canesi
-
Article |
 A bridged backbone strategy enables collective synthesis of strychnan alkaloids
A bridged backbone strategy enables collective synthesis of strychnan alkaloids
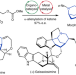
The morphan core is central to strychnan alkaloid synthesis and is typically formed during the middle or later stages of the process. Now it has been shown that an allene/ketone-equipped morphan core can be constructed early in the synthesis through ketone α-allenylation and then used to introduce other rings and functionalities, enabling access to nine targets including strychnine and geissolosimine.
- Wenqiang Zhou
- , Song Xi
- & Min Zhang
-
Article |
 Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
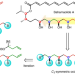
Polyketide natural products often contain common repeat motifs that are synthesized using iterative processes. Now a masked 1,3-diol motif, generated by a two-step process based on boronic ester homologation, has enabled the efficient iterative synthesis of polyacetates, including bahamaolide A. In addition to oxidation, the 1,3-polyboronic esters were shown to undergo various stereospecific transformations.
- Sheenagh G. Aiken
- , Joseph M. Bateman
- & Varinder K. Aggarwal
-
Article |
 Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Tigilanol tiglate is a therapeutic lead for the treatment of a broad range of cancers. Now, it has been shown that tigilanol tiglate can be synthesized in a time and step economical fashion from phorbol—its naturally abundant biosynthetic precursor. This synthesis provides rapid access to analogues with unprecedented protein kinase C binding activity.
- Paul A. Wender
- , Zachary O. Gentry
- & Edward Njoo
-
Article |
 Total synthesis of structurally diverse pleuromutilin antibiotics
Total synthesis of structurally diverse pleuromutilin antibiotics
General synthetic methods to access pleuromutilin antibiotics are limited due to their complex carbocyclic skeleton. Now, a synthetic platform has been developed to access structurally diverse pleuromutilins with variations at the quaternary C12 position and hydrindanone cores. Seventeen structurally distinct derivatives were prepared and evaluated against a panel of Gram-positive and -negative bacteria.
- Olivia Goethe
- , Mikaela DiBello
- & Seth B. Herzon
-
Article |
 Synthesis and single-molecule imaging reveal stereospecific enhancement of binding kinetics by the antitumour eEF1A antagonist SR-A3
Synthesis and single-molecule imaging reveal stereospecific enhancement of binding kinetics by the antitumour eEF1A antagonist SR-A3
The total synthesis and complete stereochemical assignment of the cyclic peptide natural product SR-A3—which has potential as a cancer therapeutic—has now been reported. Single-molecule biophysical and cellular experiments reveal a crucial, stereospecific role for a side-chain hydroxyl in SR-A3, which confers enhanced target residence time and efficacy in a mouse tumour model.
- Hao-Yuan Wang
- , Haojun Yang
- & Jack Taunton
-
Article |
 Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
In silico chemical prediction of a polyketide synthase gene cluster in the bacterium Gynuella sunshinyii has led to the discovery of a class of natural products called janustatins. The absolute configuration of the stereocentres in these compounds was determined by a combination of techniques including DFT calculations and 2D NMR experiments—and finally confirmed by total synthesis. Janustatins were found to cause delayed, synchronized cell death at subnanomolar concentrations.
- Reiko Ueoka
- , Philipp Sondermann
- & Jörn Piel
-
Article |
 Chemoenzymatic synthesis of fluorinated polyketides
Chemoenzymatic synthesis of fluorinated polyketides
The introduction of fluorine into a drug molecule can alter the biological responses to it, including modulating bioavailability, pharmacokinetics and selectivity. Now, a hybrid polyketide/fatty acid synthase multienzyme has been designed to incorporate fluorinated precursors during polyketide biosynthesis in an approach that provides new chemoenzymatic access to fluorinated natural compounds.
- Alexander Rittner
- , Mirko Joppe
- & Martin Grininger
-
Article |
 Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy
Total synthesis of nine longiborneol sesquiterpenoids using a functionalized camphor strategy
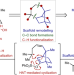
Generating strategies that use modern synthetic methods to access privileged chemical space is a central goal of total synthesis. Now, a strategy that is orthogonal to classic approaches, coupled with C–H functionalization methods, leads to the shortest synthesis of the sesquiterpenoid longiborneol and divergent syntheses of oxygenated longiborneol congeners.
- Robert F. Lusi
- , Goh Sennari
- & Richmond Sarpong
-
In Your Element |
 Quinine fever
Quinine fever
John Woodland and Kelly Chibale retrace the tumultuous history of quinine from a medicine — used as a tool for colonialism — to a puzzling chemical target, a fluorescence standard and a key ingredient in popular drinks.
- John G. Woodland
- & Kelly Chibale
-
Article |
 Concise, scalable and enantioselective total synthesis of prostaglandins
Concise, scalable and enantioselective total synthesis of prostaglandins
Current methods for the synthesis of prostaglandins suffer from low yields and lengthy steps. Now, a strategy for their enantioselective synthesis has been developed with rhodium-catalysed enyne cycloisomerization as the key step. This concise route was scaled up, enabling the preparation of fluprostenol on a 20-gram scale.
- Fuhao Zhang
- , Jingwen Zeng
- & Xumu Zhang
-
Article |
 Expanding the antibacterial selectivity of polyether ionophore antibiotics through diversity-focused semisynthesis
Expanding the antibacterial selectivity of polyether ionophore antibiotics through diversity-focused semisynthesis
Polyether ionophores are natural products that display antibacterial activity—but they also show activity against mammalian cells, which has limited their development as clinical antibiotics. Now, a semisynthesis principle of recycling substructures from highly abundant natural polyether ionophores has been used to prepare analogues with enhanced selectivity towards bacterial cells.
- Shaoquan Lin
- , Han Liu
- & Thomas B. Poulsen
-
News & Views |
 Making light work of lignan synthesis
Making light work of lignan synthesis
Mimicking biosynthetic pathways can provide access to medicinally important natural products, but generating the reactive species used by nature can often be difficult. Now, a photoredox-based strategy has been developed to access a reactive radical intermediate postulated to be involved in complex lignan biosynthesis.
- Danny Q. Thach
- & Thomas J. Maimone
-
Article |
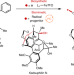 Mimicking oxidative radical cyclizations of lignan biosynthesis using redox-neutral photocatalysis
Mimicking oxidative radical cyclizations of lignan biosynthesis using redox-neutral photocatalysis
Many biosynthetic cyclizations are catalysed by iron oxygenases and appear to involve long-lived radical species. Now, mimicking these biosynthetic transformations, the total synthesis of highly oxidized lignan natural products has been reported using redox-neutral photocatalysis to enable late-stage radical cyclizations that install challenging 5- and 11-membered rings.
- Zheng Huang
- & Jean-Philip Lumb
-
Article |
 Structural snapshots of the minimal PKS system responsible for octaketide biosynthesis
Structural snapshots of the minimal PKS system responsible for octaketide biosynthesis
The invariable core of a type II polyketide synthase has been characterized using X-ray crystallography, simulations, mutagenesis experiments and functional assays. The characterization of the ternary acyl carrier protein complexes provides a mechanistic understanding of the reactivity and could inform future engineering of this complex biosynthetic machinery.
- Alois Bräuer
- , Qiuqin Zhou
- & Michael Groll
-
Article |
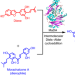 FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis
FAD-dependent enzyme-catalysed intermolecular [4+2] cycloaddition in natural product biosynthesis
A naturally occurring stand-alone and intermolecular Diels–Alderase, MaDA, has been identified from Morus alba cell cultures. MaDA is a FAD-dependent enzyme, which catalyses the intermolecular [4+2] cycloaddition via a concerted but asynchronous pericyclic pathway between morachalcone A and a diene generated from moracin C. Characterization revealed that MaDA possesses good substrate promiscuity towards both dienes and dienophiles.
- Lei Gao
- , Cong Su
- & Xiaoguang Lei
-
Article |
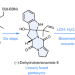 Total synthesis of brevianamide A
Total synthesis of brevianamide A
Despite five decades of research, the alkaloid (+)-brevianamide A has remained an elusive target for chemical synthesis. Now, it has been shown that the total synthesis of (+)-brevianamide A can be achieved in seven steps and 7.2% overall yield to give 750 mg of the target compound.
- Robert C. Godfrey
- , Nicholas J. Green
- & Andrew L. Lawrence
-
Article |
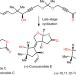 Total synthesis of terpenes via palladium-catalysed cyclization strategy
Total synthesis of terpenes via palladium-catalysed cyclization strategy
Inverting the order of nature’s two-phase biosynthesis of terpenes offers a strategy by which the synthesis of these compounds can be simplified. The key reaction is a palladium-catalysed polyenyne cycloisomerization that not only tolerates the presence of all of the oxygen functionalities but also is facilitated by them.
- Barry M. Trost
- & Chang Min
-
Article |
 Electronic complementarity permits hindered butenolide heterodimerization and discovery of novel cGAS/STING pathway antagonists
Electronic complementarity permits hindered butenolide heterodimerization and discovery of novel cGAS/STING pathway antagonists
Although biaryl rings can be easily formed via cross coupling, their tetrahedral, high-fraction sp3 equivalents cannot. Now the scope, mechanism and biological profile of a general attached-ring synthesis has been probed. This provides direct access to full bridgehead substitution via sp3–sp3 coupling and enables rapid entry to natural product space.
- Benjamin J. Huffman
- , Shuming Chen
- & Ryan A. Shenvi
-
News & Views |
 A radical approach to diverse meroterpenoids
A radical approach to diverse meroterpenoids
Meroterpenoids are mixed terpenoid–polyketide natural products with a variety of biological activities. Now, a synthetic approach that combines biocatalytic oxidation with a range of other radical-based reactions enables the divergent synthesis of eight oxidized meroterpenoid natural products and one analogue.
- Andrew Gomm
- & Adam Nelson
-
Article |
 Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids
Merging chemoenzymatic and radical-based retrosynthetic logic for rapid and modular synthesis of oxidized meroterpenoids
Meroterpenoids are mixed terpenoid–polyketide natural products that exhibit a range of biological activities. A hybrid synthetic strategy that combines biocatalytic and radical-based methods has now been developed and it enables eight different oxidized meroterpenoids to be made in just 7–12 steps from commercially available materials.
- Jian Li
- , Fuzhuo Li
- & Hans Renata
-
News & Views |
 The mysteries of macrocyclic colibactins
The mysteries of macrocyclic colibactins
The biosynthetic pathway that produces the structurally uncharacterized gut bacterial genotoxin colibactin can produce unstable, macrocyclic products; however, the extent to which these structures contribute to colibactin’s biological activities is not yet fully understood. Now, two recent studies have provided new insights and reached distinct conclusions regarding their potential mechanisms of action and relevance for genotoxicity.
- Erik S. Carlson
- & Emily P. Balskus
-
Article |
 Synthesis and reactivity of precolibactin 886
Synthesis and reactivity of precolibactin 886
Precolibactin 886 is a complex microbiome-derived metabolite implicated in colorectal cancer and produced by the clb gene cluster. A chemical synthesis and analysis of precolibactin 886 is reported which shows that its biosynthetic precursor degrades to other known clb metabolites. The data also provide insights into the structures and reactivity of advanced clb products.
- Alan R. Healy
- , Kevin M. Wernke
- & Seth B. Herzon
-
News & Views |
 Simple start for complex products
Simple start for complex products
Natural products often provide lead scaffolds for the development of therapeutics, but complexity of their synthesis can limit the discovery of improved analogues. Pharmacophore-directed retrosynthesis aims to accelerate the building of a structure–activity relationship profile of a natural product, aiming to identifying a simplified lead.
- Jason R. Hudlicky
- & Gary A. Sulikowski
-
Article |
 Simplified immunosuppressive and neuroprotective agents based on gracilin A
Simplified immunosuppressive and neuroprotective agents based on gracilin A
Pharmacophore-directed retrosynthesis targets a potential pharmacophore from early on in a natural product synthesis and incremental increases in the complexity of this minimal structure enable a SAR profile to develop over the course of the campaign. The method is applied to gracilin A, finding simplified derivatives displaying potent immunosuppressive effects or selective neuroprotective effects in cell-based assays.
- Mikail E. Abbasov
- , Rebeca Alvariño
- & Daniel Romo
-
Article |
 Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3
Chromopynones are pseudo natural product glucose uptake inhibitors targeting glucose transporters GLUT-1 and -3
New natural-product-inspired molecules are often limited by their only partial coverage of biologically relevant chemical space. Combining fragments of natural products has now been shown to yield pseudo natural products, which — while still being inspired by natural products — populate previously unexplored areas of chemical space and have novel biological activities.
- George Karageorgis
- , Elena S. Reckzeh
- & Herbert Waldmann
-
Article |
 Isolation, synthesis and bioactivity studies of phomactin terpenoids
Isolation, synthesis and bioactivity studies of phomactin terpenoids
Natural product chemistry remains critical to the discovery of small molecules that possess unique bioactivities. A collaborative approach to studying the phomactin diterpenoid family that spans isolation, chemical synthesis and investigation of their bioactivity is now reported. The novel congeners that were isolated inspired a divergent strategy to achieve their practical preparation and their anti-tumour evaluation.
- Yusuke Kuroda
- , Karen J. Nicacio
- & Richmond Sarpong
-
Article |
 Direct α-C–H bond functionalization of unprotected cyclic amines
Direct α-C–H bond functionalization of unprotected cyclic amines
Cyclic amines bearing α-substituents are valuable building blocks for drug discovery and natural product synthesis. Introduction of α-substituents via site-selective replacement of C–H bonds is highly attractive but typically limited to protected amine substrates. Now, an operationally simple hydride-transfer-based approach enables the introduction of α-substituents on unprotected amines.
- Weijie Chen
- , Longle Ma
- & Daniel Seidel
-
Article |
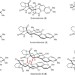 Bioinspired chemical synthesis of monomeric and dimeric stephacidin A congeners
Bioinspired chemical synthesis of monomeric and dimeric stephacidin A congeners
The biosynthesis of secondary metabolites such as stephacidin A and its congeners continues to intrigue both biochemists and synthetic chemists. Now, a laboratory chemical synthesis of these natural products has been achieved based on a bioinspired synthetic strategy, which may provide key insights into the possible biosynthesis of these captivating molecules.
- Ken Mukai
- , Danilo Pereira de Sant'Ana
- & Richmond Sarpong
-
Article |
 Catalyst-controlled oligomerization for the collective synthesis of polypyrroloindoline natural products
Catalyst-controlled oligomerization for the collective synthesis of polypyrroloindoline natural products
The collective synthesis of several oligomeric polypyrroloindoline natural products, including hodgkinsine, hodgkinsine B, idiospermuline, quadrigemine H and isopsychotridines B and C, is accomplished through the iterative action of an asymmetric small molecule copper catalyst. This strategy also enables the synthesis of putatively unnatural quadrigemine H-type isomers.
- Christopher R. Jamison
- , Joseph J. Badillo
- & David W. C. MacMillan
-
Article |
 Synthesis facilitates an understanding of the structural basis for translation inhibition by the lissoclimides
Synthesis facilitates an understanding of the structural basis for translation inhibition by the lissoclimides
Several natural and unnatural lissoclimide cytotoxins have been prepared via semi-synthesis and total synthesis. An X-ray co-crystal structure of chlorolissoclimide with the ribosome and evaluation of cytotoxicity and translation inhibition of new compounds in the series improves our understanding of the molecular basis for cytotoxicity.
- Zef A. Könst
- , Anne R. Szklarski
- & Christopher D. Vanderwal
-
Article |
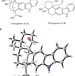 Total synthesis of (–)-tubingensin B enabled by the strategic use of an aryne cyclization
Total synthesis of (–)-tubingensin B enabled by the strategic use of an aryne cyclization
The total synthesis of tubingensin B — an indole diterpenoid that bears a daunting chemical structure — has now been achieved. The design and evolution of this succinct total synthesis underscores the utility of long-avoided aryne intermediates for the introduction of structural motifs that have conventionally been viewed as challenging.
- Michael A. Corsello
- , Junyong Kim
- & Neil K. Garg
-
News & Views |
 Taming reactive benzynes
Taming reactive benzynes
Natural products often serve as sources of new drugs, either directly or after synthetic modification, but site-selective functionalization of complex small molecules is challenging. Now, a method has been developed that enables selective modification of a wide range of natural products by engaging a benzyne intermediate in a variety of reaction modes.
- Sarah Z. Tasker
- & Paul J. Hergenrother
-
Article |
 Unified biomimetic assembly of voacalgine A and bipleiophylline via divergent oxidative couplings
Unified biomimetic assembly of voacalgine A and bipleiophylline via divergent oxidative couplings
The biomimetic syntheses of bipleiophylline, one of the most complex monoterpene indole alkaloids, and voacalgine A, its biosynthetic precursor, have been achieved from pleiocarpamine starting material. The development of a divergent oxidative coupling for the formation of the benzofuro[2,3-b]indolenine and isochromano[3,4-b]indolenine moieties was key to this accomplishment.
- David Lachkar
- , Natacha Denizot
- & Guillaume Vincent
-
News & Views |
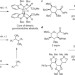 A radical step forward
A radical step forward
Free radicals are notorious for unselective coupling reactions; however, the coupling of free radicals generated from acyl tellurides has now been shown to form C–C bonds with remarkable fidelity, which enables easy one-step assembly of densely oxygenated natural product motifs.
- Wenhao Zhang
- & Ang Li
-
Article |
 Synthesis of ent-BE-43547A1 reveals a potent hypoxia-selective anticancer agent and uncovers the biosynthetic origin of the APD-CLD natural products
Synthesis of ent-BE-43547A1 reveals a potent hypoxia-selective anticancer agent and uncovers the biosynthetic origin of the APD-CLD natural products
Anti-proliferative compounds that display enhanced toxicity in a low-oxygen (hypoxic) environment may be used to eradicate aggressive and therapy-resistant cancer cells. Now, a promising lead structure has been identified in the BE-43547-class of depsipeptide natural products.
- Nikolaj L. Villadsen
- , Kristian M. Jacobsen
- & Thomas B. Poulsen
-
Article |
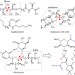 Direct assembly of multiply oxygenated carbon chains by decarbonylative radical–radical coupling reactions
Direct assembly of multiply oxygenated carbon chains by decarbonylative radical–radical coupling reactions
Pentoses and hexoses represent important structural motifs in bioactive secondary metabolites, though their synthesis often requires several elongation steps. Now, a method for radical–radical coupling reactions of sugar derivatives enables the single-step preparation of the oxygenated carbon chains of several natural products, including sagittamide D, maitotoxin and hikizimycin.
- Kengo Masuda
- , Masanori Nagatomo
- & Masayuki Inoue
-
News & Views |
 A light touch breaks a strong ring
A light touch breaks a strong ring
The high stability of aromatic compounds often limits the types of reaction that can be conducted on them. Now, a series of photochemically promoted addition reactions has been used to effect the oxidative dearomatization of benzene derivatives. These reactions provide a suite of versatile new building blocks for chemical synthesis.
- Martin G. Banwell
-
Article |
 Dearomative dihydroxylation with arenophiles
Dearomative dihydroxylation with arenophiles
Dearomatization reactions that can simultaneously introduce functionality are valuable transformations that are largely underdeveloped. Now, a synthetic strategy based on the combination of arenophiles with catalytic dihydroxylation reactions enables rapid and controlled access to synthetically useful cyclohexene and cyclohexadiene derivatives from readily available arene starting materials.
- Emma H. Southgate
- , Jola Pospech
- & David Sarlah
-
Article |
 Total synthesis and structure–activity relationship studies of a series of selective G protein inhibitors
Total synthesis and structure–activity relationship studies of a series of selective G protein inhibitors
G proteins are the key mediators of G protein-coupled receptor signalling, facilitating a number of important physiological processes. Now, the total synthesis and structure–activity relationship studies have been reported for the only known selective Gq protein inhibitors, the natural cyclic depsipeptides YM-254890 and FR900359.
- Xiao-Feng Xiong
- , Hang Zhang
- & Kristian Strømgaard
-
Article |
 An antibacterial vaccination strategy based on a glycoconjugate containing the core lipopolysaccharide tetrasaccharide Hep2Kdo2
An antibacterial vaccination strategy based on a glycoconjugate containing the core lipopolysaccharide tetrasaccharide Hep2Kdo2
The presence and linkage of unusual higher sugars in the ‘inner core’ of Gram-negative bacteria makes the core lipopolysacchride tetrasaccharide Hep2Kdo2 a tough target. Now, a 2+2 glycosylation strategy has facilitated the synthesis of this glycoconjugate. Synthesis of Hep2Kdo2 enabled an antibacterial vaccination strategy based on immunization with the glycoconjugate and the subsequent administration of an inhibitor that uncovers the corresponding epitope in pathogenic bacteria.
- Lingbing Kong
- , Balakumar Vijayakrishnan
- & Benjamin G. Davis
-
News & Views |
 Emulation illuminates biosynthesis
Emulation illuminates biosynthesis
A concise synthesis of the fungal natural product epicolactone suggests that this highly stereochemically complex yet racemic natural product may come from a cascade reaction between two polyhydroxylated arenes.
- Jaron A. M. Mercer
- & Noah Z. Burns
-
Article |
 Synthesis of marmycin A and investigation into its cellular activity
Synthesis of marmycin A and investigation into its cellular activity
Marmycin A is an anthraquinone natural product with antiproliferative properties. Now the chemical synthesis of marmycin A—through a Diels–Alder cycloaddition, an Ullmann aromatic amination and a Friedel–Crafts cyclization—has enabled a study of its biological activity. Fluorescence microscopy reveals that marmycin A accumulates in lysosomes and promotes cell death independently of genome targeting.
- Tatiana Cañeque
- , Filipe Gomes
- & Raphaël Rodriguez
-
News & Views |
 Terpenes in tight spaces
Terpenes in tight spaces
The ability of enzymes to direct the synthesis of complex natural products from simple starting materials is epitomized by terpene biosynthesis. Now, a supramolecular catalyst has been shown to mimic some of the reactivity of this process.
- Jeremy J. Roach
- & Ryan A. Shenvi
-
Article |
 Pseudopterosin synthesis from a chiral cross-conjugated hydrocarbon through a series of cycloadditions
Pseudopterosin synthesis from a chiral cross-conjugated hydrocarbon through a series of cycloadditions
The pseudopterosins are a family of natural products whose interesting anti-inflammatory and pain-relieving properties have inspired many synthetic approaches. Now, an unusual approach that starts with an axially chiral hydrocarbon that engages in a triple Diels–Alder sequence has been shown to result in the shortest total synthesis of a pseudopterosin so far.
- Christopher G. Newton
- , Samuel L. Drew
- & Michael S. Sherburn
-
Article |
 Synthesis of hydroxyphthioceranic acid using a traceless lithiation–borylation–protodeboronation strategy
Synthesis of hydroxyphthioceranic acid using a traceless lithiation–borylation–protodeboronation strategy
Coupling of carbamates with boronic esters followed by protodeboronation creates a new carbon–carbon bond, leaving behind no trace of the functional groups used to create it. Now, methodology for the protodeboronation of alkyl pinacol boronic esters has been developed and an iterative lithiation–borylation–protodeboronation strategy used in a 14-step stereocontrolled synthesis of hydroxyphthioceranic acid.
- Ramesh Rasappan
- & Varinder K. Aggarwal
-
News & Views |
 Natural products on demand
Natural products on demand
Nature assembles complex natural products using bifunctional building blocks and a mere handful of reaction types. Mimicry of this method seeks to revolutionize natural product and small-molecule synthesis.
- Lawrence G. Hamann
-
Article |
 Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction
Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction
Polyene motifs are found in a large number of natural products. Now, by applying a general retrosynthetic algorithm, it has been shown that the polyene motifs found in >75% of these compounds can be synthesized using just 12 bifunctional haloalkenyl MIDA boronate building blocks and one coupling reaction.
- Eric M. Woerly
- , Jahnabi Roy
- & Martin D. Burke
-
Article |
 Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate
Simultaneous structure–activity studies and arming of natural products by C–H amination reveal cellular targets of eupalmerin acetate
Natural products are enduring leads for exploring cell biology, yet structure–activity relationship studies and 'arming' of these small molecules for subsequent cellular probe synthesis remains a challenge. Here, a strategy for derivatization of natural products by C–H amination, aziridination and unusual N-aminations is described. Selective derivatization of eupalmarin acetate led to identification of this natural product's target.
- Jing Li
- , Justin S. Cisar
- & Daniel Romo

 Modular chemoenzymatic synthesis of ten fusicoccane diterpenoids
Modular chemoenzymatic synthesis of ten fusicoccane diterpenoids