Featured
-
-
News & Views |
 A discrete antimony(v) oxide
A discrete antimony(v) oxide
Monomeric stibine oxide has remained elusive due to the large antimony orbitals coupled with a high electronegativity difference with oxygen. Now, a free tris(2,6-diisopropylphenyl)stibine oxide has been isolated that can act as oxo-transfer reagent.
- Moumita Majumdar
-
News & Views |
 Stable organolithium gels
Stable organolithium gels
Organolithium reagents are characterized by their high reactivity towards air and moisture, traditionally requiring strict inert conditions for their handling and utilization. Now, these reagents can be encapsulated within an organogel, enhancing their stability and allowing their use and storage under ambient conditions.
- Andreu Tortajada
- & Eva Hevia
-
Article
| Open Access Organogel delivery vehicles for the stabilization of organolithium reagents
Organogel delivery vehicles for the stabilization of organolithium reagents
Organolithium compounds are important reagents but are often hazardous due to their high reactivity. Now, encapsulating organolithium reagents within a supramolecular organogel has been found to enhance their stability, facilitating their storage and handling under ambient conditions. The homogeneous gels can be easily subdivided and dosed into a wide range of synthetic reactions.
- Petr Slavík
- , Benjamin R. Trowse
- & David K. Smith
-
Article |
 Taming phosphorus mononitride
Taming phosphorus mononitride
Precursors for the release of phosphorus mononitride in solution under mild conditions have remained elusive. Now, an explosive anthracene-stabilized azidophosphine has shown PN transfer reactivity in the synthesis of an Fe–NP complex. The PN ligand is N-bonded, as the Fe–N interaction shows significant covalent character and a less unfavourable Pauli repulsion than its Fe–P counterpart.
- André K. Eckhardt
- , Martin-Louis Y. Riu
- & Christopher C. Cummins
-
News & Views |
 White phosphorus first
White phosphorus first
Many of the methods used to make phosphines proceed via phosphorus trichloride-based intermediates, leading to chloride waste that is difficult to recycle. It has now been shown that this disadvantage can be overcome by using a method that directly converts white phosphorus into value-added phosphorus compounds.
- Hansjörg Grützmacher
-
Article |
 Direct conversion of white phosphorus to versatile phosphorus transfer reagents via oxidative onioation
Direct conversion of white phosphorus to versatile phosphorus transfer reagents via oxidative onioation
White phosphorus (P4) is selectively transformed by oxidative onioation into salts of P1-transfer reagents that feature reactive P–N bonds. These P1 compounds can be used in P–N, P–O and P–C bond-forming reactions to form value-added phosphorus chemicals, representing a viable alternative to the most relevant, yet problematic, P(III) precursor, namely PCl3.
- Maximilian Donath
- , Kai Schwedtmann
- & Jan J. Weigand
-
News & Views |
 Building bridges
Building bridges
Methods for the synthesis of bicyclo[1.1.1]pentanes (BCPs) typically rely on the release of strain energy, but these routes cannot easily introduce substituents on the BCP bridges. Now, an intramolecular coupling gives access to multi-substituted BCPs, through the synthesis of bridge-substituted bicycloalkyl boronic esters.
- Cara E. Brocklehurst
- & Edward A. Anderson
-
Article |
 An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates
An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates
Bicyclic hydrocarbons, and bicyclo[1.1.1]pentanes in particular, are playing an emerging role as saturated bioisosteres in pharmaceutical, agrochemical and materials intramolecular coupling approach has been developed for the modular construction of underexplored multisubstituted strained bicyclic hydrocarbons, ranging from [1.1.1] to [3.2.1] scaffolds.
- Yangyang Yang
- , Jet Tsien
- & Tian Qin
-
Article |
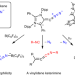 Isolation and reactivity of an elusive diazoalkene
Isolation and reactivity of an elusive diazoalkene
Although diazoalkenes have been reported as reactive intermediates in organic chemistry, their detection and isolation remains challenging. Such species have previously only been detected at low temperatures in matrix-isolation studies. Now, a room-temperature stable diazoalkene has been reported, which shows dual-site nucleophilicity and can undergo N2 exchange or lose dinitrogen under irradiation.
- P. W. Antoni
- , C. Golz
- & M. M. Hansmann
-
Article |
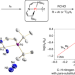 A platinum(ii) metallonitrene with a triplet ground state
A platinum(ii) metallonitrene with a triplet ground state
Transient metallonitrenes (M–N) have been proposed as key intermediates in nitrogen atom transfer reactions, but well-defined examples have remained elusive. Now, a platinum complex with an atomic nitrogen ligand, best described as a subvalent nitrogen diradical singly bonded to a platinum(ii) ion (Pt–N), has been isolated and shows ambiphilic reactivity.
- Jian Sun
- , Josh Abbenseth
- & Sven Schneider
-
Article |
 A silicon–carbonyl complex stable at room temperature
A silicon–carbonyl complex stable at room temperature
Compounds of main-group elements rarely undergo direct carbonylation reactions. Now, an electron-rich silylene intermediate has been shown to readily react with CO to form a silylene carbonyl complex that is stable at room temperature. This complex engages in CO substitution as well as oxidative addition reactions.
- Chelladurai Ganesamoorthy
- , Juliane Schoening
- & Stephan Schulz
-
Article |
 Isolation, characterization and reactivity of three-coordinate phosphorus dications isoelectronic to alanes and silylium cations
Isolation, characterization and reactivity of three-coordinate phosphorus dications isoelectronic to alanes and silylium cations
The isoelectronic series of alanes [R3Al] and silylium cations [R3Si]+ has now been extended with the synthesis and characterization of two phosphorandiylium dications ([R3P]2+), which are trigonal planar Lewis superacids. The electrophilicity at the phosphorus atom is governed by the π-electron-donating ability of the attached N-heterocyclic imine substituents.
- Paul Mehlmann
- , Tim Witteler
- & Fabian Dielmann
-
Article |
 A crystalline monosubstituted carbene
A crystalline monosubstituted carbene
So far, monosubstituted carbenes have only been spectroscopically characterized at very low temperatures. Now, it has been shown that a bulky, chemically inert, amino substituent is enough to tame the intrinsic tendency of carbenes towards dimerization, enabling their isolation at room temperature.
- Ryo Nakano
- , Rodolphe Jazzar
- & Guy Bertrand
-
Article |
 Diels–Alder cycloadditions of strained azacyclic allenes
Diels–Alder cycloadditions of strained azacyclic allenes
Strained organic compounds have long fascinated the chemistry community. Heterocyclic allenes are particularly interesting strained intermediates, but their use in synthetic chemistry is rather scarce. Now, an experimental and computational study of azacyclic allenes demonstrates that heteroatom-containing cyclic allenes can be harnessed for the construction of complex molecular scaffolds, including those that bear multiple stereogenic centres.
- Joyann S. Barber
- , Michael M. Yamano
- & Neil K. Garg
-
News & Views |
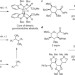 A radical step forward
A radical step forward
Free radicals are notorious for unselective coupling reactions; however, the coupling of free radicals generated from acyl tellurides has now been shown to form C–C bonds with remarkable fidelity, which enables easy one-step assembly of densely oxygenated natural product motifs.
- Wenhao Zhang
- & Ang Li

 Structural characterization and reactivity of a room-temperature-stable, antiaromatic cyclopentadienyl cation salt
Structural characterization and reactivity of a room-temperature-stable, antiaromatic cyclopentadienyl cation salt