Article
|
Open Access
Featured
-
-
Article |
 A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media
A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media
The reversible N–H activation and catalytic transformations of ammonia are a challenge. Now, a hidden frustrated Lewis pair is shown to activate non-aqueous ammonia thermoneutrally and split the N–H bond reversibly at ambient temperature. The N–H-activated ammonia was also utilized as an atom-economical nitrogen source for catalytic NH3 transfer reactions.
- Felix Krämer
- , Jan Paradies
- & Frank Breher
-
News & Views |
 Hydrogenative double-bond deuteration
Hydrogenative double-bond deuteration
Deuterated compounds are used in many applications such as mass-spectrometry standards, drugs or in organic light-emitting diodes. Now, hydrogen-activated homogeneous pincer complex catalysts can be used to perform selective alkene deuteration with the cheapest available deuterium source, D2O.
- Anika Tarasewicz
- & Volker Derdau
-
News & Views |
 Aryl ortho methoxylation
Aryl ortho methoxylation
Aryl ethers are useful intermediates in organic synthesis and are found in countless biologically active compounds. Now, through palladium/norbornene cooperative catalysis and incorporation of a polarity-reversed N–O reagent as the O-electrophile, an efficient arene methoxylation approach has been successfully developed.
- Kun Zhao
- & Zhenhua Gu
-
Article |
 Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Carbonyl cross-metathesis via deoxygenative gem-di-metal catalysis
Merging carbonyls to form an alkene by removing their oxygens is rare, yet synthetically useful, and the selective combination of two different carbonyls is especially challenging. Now, two strategies for cross-metathesis of unbiased carbonyls have been developed. An Fe-catalysed carbene/ylide strategy affords Z-alkenes, while Cr-catalysed gem-di-metals yield E-alkenes.
- Lumin Zhang
- & David A. Nagib
-
Article |
 Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Cobalt(II) complexes of porphyrins have dominated the development of metalloradical catalysts. Now it has been shown that five-coordinate iron(III) complexes of porphyrins with an axial ligand are also potent metalloradical catalysts for olefin cyclopropanation. They are shown to react with different classes of diazo compounds via a stepwise radical mechanism.
- Wan-Chen Cindy Lee
- , Duo-Sheng Wang
- & X. Peter Zhang
-
Article |
 Hydrogenative alkene perdeuteration aided by a transient cooperative ligand
Hydrogenative alkene perdeuteration aided by a transient cooperative ligand
Deuterogenation methods typically introduce only two deuterium atoms per unsaturation. Now the single-step hydrogenative perdeuteration of alkenes has been achieved using H2 and D2O, with incorporation of up to 4.9 D atoms per C=C double bond. The reaction is catalysed by a ruthenium pincer complex with a catalytic amount of thiol, which serves as a transient cooperative ligand.
- Jie Luo
- , Lijun Lu
- & David Milstein
-
Article |
 Ortho-C–H methoxylation of aryl halides enabled by a polarity-reversed N–O reagent
Ortho-C–H methoxylation of aryl halides enabled by a polarity-reversed N–O reagent
Pd/norbornene cooperative catalysis provides a strategy for arene functionalization, but the electrophile scope is typically limited to ‘soft’ elements such as carbon, nitrogen and sulfur. Now the ortho-C–H methoxylation of aryl halides has been realized using a polarity-reversed N−O reagent and facilitated by a C7-bromo-substituted norbornene mediator.
- Xin Liu
- , Yue Fu
- & Guangbin Dong
-
Article
| Open Access Access to unsaturated bicyclic lactones by overriding conventional C(sp3)–H site selectivity
Access to unsaturated bicyclic lactones by overriding conventional C(sp3)–H site selectivity
Bicyclic lactones are valuable motifs for the synthesis of natural products and bioactive molecules. Now, a palladium-catalysed protocol has been developed to access unsaturated bicyclic lactones in one step from corresponding carboxylic acids. The method demonstrates reverse site selectivity for C(sp3)–H activation to form diverse bicyclic cores.
- Jayabrata Das
- , Wajid Ali
- & Debabrata Maiti
-
Article |
 Nucleation-mediated growth of chiral 3D organic–inorganic perovskite single crystals
Nucleation-mediated growth of chiral 3D organic–inorganic perovskite single crystals
While chiral hybrid organic–inorganic perovskites are promising materials for optoelectronic applications, the synthesis of three-dimensional single crystals has proven challenging. Now, a general strategy has been shown to synthesize chiral, three-dimensional perovskites by heterogeneous nucleation. The single-crystalline materials contain no chiral component; their chiroptical activity arises from supercells formed by chiral patterns of the A-site cations.
- Gaoyu Chen
- , Xiaoyu Liu
- & Xun Wang
-
News & Views |
 Total synthesis of strychnan alkaloids
Total synthesis of strychnan alkaloids
The synthesis of natural products with important biological properties has always fascinated chemists, but the development of rapid, efficient and stereoselective transformations remains challenging. Now, a strategy has been developed to produce several strychnan alkaloids through formation of a common bridged morphan core structure.
- Sylvain Canesi
-
News & Views |
 Diradical ring formation
Diradical ring formation
Lactams and pyridines are privileged scaffolds, but strategies for combining these groups into one molecule are lacking. Now, N–N pyridinium ylides have been shown to form triplet state diradicals under photoinduced energy transfer, and subsequent [3+2] cycloaddition with the tethered alkene enables the synthesis of diverse ortho-pyridyl lactams.
- Peng-Zi Wang
- & Jia-Rong Chen
-
Article |
 A bridged backbone strategy enables collective synthesis of strychnan alkaloids
A bridged backbone strategy enables collective synthesis of strychnan alkaloids
The morphan core is central to strychnan alkaloid synthesis and is typically formed during the middle or later stages of the process. Now it has been shown that an allene/ketone-equipped morphan core can be constructed early in the synthesis through ketone α-allenylation and then used to introduce other rings and functionalities, enabling access to nine targets including strychnine and geissolosimine.
- Wenqiang Zhou
- , Song Xi
- & Min Zhang
-
Article |
 Energy-transfer-induced [3+2] cycloadditions of N–N pyridinium ylides
Energy-transfer-induced [3+2] cycloadditions of N–N pyridinium ylides
There is currently a lack of effective synthetic strategies for combining lactams and pyridines within a single molecular structure. Now, diastereoselective pyridyl lactamization has been developed using a photoinduced [3+2] cycloaddition of triplet diradical N–N pyridinium ylides with pendant alkenes. This method provides a useful synthon for preparing pyridyl γ- and δ-lactam scaffolds with syn-configuration.
- Wooseok Lee
- , Yejin Koo
- & Sungwoo Hong
-
Article |
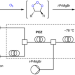 Capturing primary ozonides for a syn-dihydroxylation of olefins
Capturing primary ozonides for a syn-dihydroxylation of olefins
Traditionally, ozone has been primarily used to oxidatively deconstruct carbon–carbon bonds. Now, it has been shown that ozone can be used for the construction of carbon–oxygen bonds without oxidative cleavage of the olefin substrate through capturing primary ozonides. Furthermore, intercepting primary ozonides with nucleophiles in continuous flow enabled the green, syn-dihydroxylation of olefins to be realized.
- Danniel K. Arriaga
- & Andy A. Thomas
-
Article |
 An electroaffinity labelling platform for chemoproteomic-based target identification
An electroaffinity labelling platform for chemoproteomic-based target identification
The covalent capture of a ligand by its target protein(s) is important for drug-target identification. Now an electrochemically active warhead—diazetidinone—can be leveraged in a chemoproteomics platform for electroaffinity labelling of a ligand’s target protein to afford target-ligand identification in live cells.
- Yu Kawamata
- , Keun Ah Ryu
- & Phil S. Baran
-
Research Briefing |
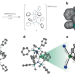 A near-linear lanthanide complex that displays magnet-like behaviour
A near-linear lanthanide complex that displays magnet-like behaviour
Low-coordinate lanthanide complexes with strong magnetic anisotropy could afford high-performance single-molecule magnets (SMMs) but are challenging to synthesize. Now, through ligand design, a near-linear pseudo-two-coordinate Yb(iii) complex that exhibits slow magnetic relaxation is reported. The complex has a large total splitting of the ground-state manifold, arising from the crystal field imposed by the ligands.
-
Article |
 Copper-catalysed difluorocarbene transfer enables modular synthesis
Copper-catalysed difluorocarbene transfer enables modular synthesis
Although several transition-metal carbene complexes have been isolated and used for catalytic carbene transfer reactions, few metal difluorocarbene complexes have been reported. Now, the synthesis, characterization and reactivity of isolable copper(I) difluorocarbene complexes has been reported, which has enabled the development of a copper-catalysed difluorocarbene transfer reaction to access fluorinated compounds from simple chemical feedstocks.
- Xin Zeng
- , Yao Li
- & Xingang Zhang
-
Article
| Open Access 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring
The ortho-substituted phenyl ring is a basic structural element in chemistry. Now, 2-oxabicyclo[2.1.1]hexanes have been developed as saturated bioisosteres of the ortho-substituted phenyl ring with improved physicochemical properties. Replacement of the phenyl ring with 2-oxabicyclo[2.1.1]hexanes in marketed agrochemicals fluxapyroxad and boscalid improved water solubility, reduced lipophilicity and retained bioactivity.
- Aleksandr Denisenko
- , Pavel Garbuz
- & Pavel K. Mykhailiuk
-
News & Views |
 Arene additions
Arene additions
Dipolar cycloadditions are excellent processes for generating heterocyclic systems from simple starting materials, but arenes as dipolarophiles have not been extensively explored. Now, the intramolecular dipolar cycloaddition of aromatic rings has been achieved using in situ generated diazoalkenes to produce bicyclic or tricyclic heterocycles.
- Abraham Ustoyev
- & Mitchell P. Croatt
-
Article |
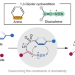 Arenes participate in 1,3-dipolar cycloaddition with in situ-generated diazoalkenes
Arenes participate in 1,3-dipolar cycloaddition with in situ-generated diazoalkenes
1,3-Dipolar cycloadditions are well-known transformations in organic synthesis. However, the reactivity of benzene rings in these processes is underexplored. In situ-generated diazoalkenes have now been shown to undergo intramolecular 1,3-dipolar cycloadditions with aromatic rings. The transformation results in an unaromatized benzene ring that enables the synthesis of functionalized heterocycles.
- Shubhangi Aggarwal
- , Alexander Vu
- & Valery V. Fokin
-
Article |
 Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
The diverse site-selective functionalization, including multi-functionalization, of C=C double bonds and C(sp3)–H bonds remains a largely unmet challenge. Now, a palladium-catalysed aerobic oxidative method has been developed for the multi-site programmable functionalization of terminal olefins via a strategy that controls the reaction sequence between alkene isomerization and oxidative functionalization.
- Zhengxing Wu
- , Jingjie Meng
- & Wanbin Zhang
-
Research Briefing |
 Not all catenanes with oriented rings are topologically chiral
Not all catenanes with oriented rings are topologically chiral
Catenanes that are chiral owing to the relative orientation of their rings have always been referred to as ‘topologically chiral’. It is now shown that although in specific cases the stereochemistry is a topological property of the structure, the underlying stereogenic unit itself is not inherently topological in nature.
-
Article
| Open Access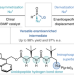 Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective nucleophilic desymmetrization of phosphonate esters
Catalytic enantioselective approaches to access chiral organophosphorus compounds are rare. A two-stage catalytic strategy for the synthesis of diverse enantioenriched P(V) compounds has now been developed: a bifunctional iminophosphorane superbase enables enantioselective nucleophilic desymmetrization, followed by downstream enantiospecific diversification of the resulting intermediate by substitution with medicinally relevant O-, N- and S-based nucleophiles.
- Michele Formica
- , Tatiana Rogova
- & Darren J. Dixon
-
Article |
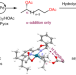 Regio- and enantioselective remote dioxygenation of internal alkenes
Regio- and enantioselective remote dioxygenation of internal alkenes
Functionalization of unsymmetrical internal alkenes usually takes place with low regioselectivity, giving a mixture of isomers. Now, a highly regio- and enantioselective remote 1,n-dioxygenation of internal alkenes using a palladium catalyst has been developed for the synthesis of chiral 1,n-diols. Regioselectivity tuning was demonstrated by altering the rate-determining step, enabled by the phenyl-substituted Pyox ligand.
- Xiaonan Li
- , Tilong Yang
- & Guosheng Liu
-
News & Views |
 Benzene-like N6 hexazine rings
Benzene-like N6 hexazine rings
The chemistry of polynitrogens has been enriched by a new isomer of N6 through the synthesis, in a laser-heated diamond anvil cell, of a charged aromatic [N6]4– ring that is recoverable at ambient temperature under high pressure.
- Sandra Ninet
-
News & Views |
 A discrete antimony(v) oxide
A discrete antimony(v) oxide
Monomeric stibine oxide has remained elusive due to the large antimony orbitals coupled with a high electronegativity difference with oxygen. Now, a free tris(2,6-diisopropylphenyl)stibine oxide has been isolated that can act as oxo-transfer reagent.
- Moumita Majumdar
-
Article |
 Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Synthesis of planar chiral ferrocenes via enantioselective remote C–H activation
Methods for the direct construction of 1,3-disubstituted planar chiral ferrocenes are elusive. Now, a modular platform enables the construction of planar chirality in 1,3-disubstituted ferrocenes/ruthenocenes via enantioselective relay remote C–H arylation. The strategy involves an initial enantiodetermining ortho-C‒H activation enabled by a Pd(II)/chiral amino-acid ligand, followed by relay to the remote meta-position by a bridgehead-substituted norbornene mediator.
- Lan Zhou
- , Hong-Gang Cheng
- & Qianghui Zhou
-
News & Views |
 Amino amide assembly
Amino amide assembly
Enantioenriched β-amino acid derivatives are attractive synthetic targets, considering the significance of these motifs in medicinal and material chemistry. Now, using ambiphilic ynamides as two-carbon synthons in a four-component reaction, three classes of β-amino amides with well-defined stereocentres can be accessed.
- Sunliang Cui
- & Linwei Zeng
-
Article |
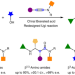 Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions
Modular enantioselective access to β-amino amides by Brønsted acid-catalysed multicomponent reactions
β-Amino acids are broadly found in biomimetic drugs and therapeutics, but the general synthesis of chiral β-amino amides remains limited. An organocatalytic four-component reaction has now been developed for their asymmetric synthesis with notable efficiency, chemoselectivity and stereoselectivity. This protocol shows broad product scope and provides a shortcut to other important structures.
- Jun Wei
- , Jian Zhang
- & Bin Tan
-
-
Article |
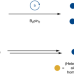 Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes
Catalytic undirected borylation of tertiary C–H bonds in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes
Borylated bicyclopentanes and bicyclohexanes are valuable compounds in drug research but are difficult to prepare. Now, an iridium-catalysed method has been developed for the borylation of the bridgehead tertiary C–H bonds in bicyclopentanes and bicyclohexanes, providing access to a variety of highly decorated bicyclic cores.
- Isaac F. Yu
- , Jenna L. Manske
- & John F. Hartwig
-
Research Briefing |
 Serial rotation electron diffraction for rapid phase analysis to accelerate materials development
Serial rotation electron diffraction for rapid phase analysis to accelerate materials development
Serial rotation electron diffraction (SerialRED) enables rapid and reliable phase analysis and structure determination of complex polycrystalline materials that cannot be routinely characterized using X-ray diffraction. Five zeolite phases were identified in a single synthesis product by automated screening of hundreds of crystals, demonstrating the power of SerialRED for materials development.
-
Article |
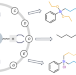 Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
Stepwise on-demand functionalization of multihydrosilanes enabled by a hydrogen-atom-transfer photocatalyst based on eosin Y
The controlled functionalization of multihydrosilanes is challenging. Now, using a hydrogen-atom-transfer photocatalyst based on neutral eosin Y, a method for the diverse functionalization of hydrosilanes has been developed, enabling the stepwise on-demand decoration of silicon atoms. This approach is distinguished by its atom-, step-, redox- and catalyst-economy, metal-free nature, its versatility (>150 examples), modularity, selectivity and scalability.
- Xuanzi Fan
- , Muliang Zhang
- & Jie Wu
-
Article |
 Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres
Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres
Direct and stereospecific C(sp3)–C(sp2) cross-coupling reactions are highly desirable for the construction of complex molecular scaffolds. Now, using stable and easily accessible alkyl sulfinates as coupling reagents, a modular and programmable sulfurane-mediated coupling method has been developed for the installation of various hindered alkyl bioisosteres, such as trifluoromethyl cyclopropyl, to (hetero)aromatics.
- Min Zhou
- , Jet Tsien
- & Tian Qin
-
News & Views |
 Stable organolithium gels
Stable organolithium gels
Organolithium reagents are characterized by their high reactivity towards air and moisture, traditionally requiring strict inert conditions for their handling and utilization. Now, these reagents can be encapsulated within an organogel, enhancing their stability and allowing their use and storage under ambient conditions.
- Andreu Tortajada
- & Eva Hevia
-
Article
| Open Access Organogel delivery vehicles for the stabilization of organolithium reagents
Organogel delivery vehicles for the stabilization of organolithium reagents
Organolithium compounds are important reagents but are often hazardous due to their high reactivity. Now, encapsulating organolithium reagents within a supramolecular organogel has been found to enhance their stability, facilitating their storage and handling under ambient conditions. The homogeneous gels can be easily subdivided and dosed into a wide range of synthetic reactions.
- Petr Slavík
- , Benjamin R. Trowse
- & David K. Smith
-
Article |
 A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres
A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres
Substituted bicyclo[2.1.1]hexanes (BCHs) are emerging bicyclic hydrocarbon bioisosteres for ortho- and meta-substituted benzenes, but are difficult to access. Now a SmI2-catalysed intermolecular coupling of bicyclo[1.1.0]butyl ketones and alkenes provides a general approach to access substituted BCHs, thus promoting their widespread use in medicinal chemistry and crop science.
- Soumitra Agasti
- , Frédéric Beltran
- & David J. Procter
-
Article
| Open Access High-throughput phase elucidation of polycrystalline materials using serial rotation electron diffraction
High-throughput phase elucidation of polycrystalline materials using serial rotation electron diffraction
X-ray diffraction is crucial for the phase elucidation of polycrystalline materials but remains challenging for complex multiphase systems. Now serial rotation electron diffraction has been shown to enable rapid, reliable and semiquantitative phase analysis of such systems, facilitating high-throughput screening of complex synthesis systems and providing new opportunities for materials development.
- Yi Luo
- , Bin Wang
- & Xiaodong Zou
-
News & Views |
 Chiral sulfinyls from sulfones
Chiral sulfinyls from sulfones
The selective removal of one oxygen atom from sulfones, without over-reduction to sulfide, is a challenging task. Now, through organocatalysis and incorporation of a cyano group into the sulfone, an asymmetric deoxygenation strategy has been developed, providing an efficient method for the synthesis of chiral sulfinyl compounds.
- Elżbieta Wojaczyńska
- & Jacek Wojaczyński
-
Article |
 Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
The direct conversion of sulfones to chiral sulfinyl compounds is one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Now, through incorporation of a cyano group into the sulfone, an organocatalytic asymmetric deoxygenation strategy has been developed that enables the synthesis of chiral sulfinyl compounds.
- Shengli Huang
- , Zhen Zeng
- & Hailong Yan
-
Article |
 Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Controlling various selectivities in radical reactions presents both formidable challenges and great opportunities. Now, Co(II)-based metalloradical catalysis has enabled the concurrent control of multiple convergences and selectivities in intermolecular radical allylic C−H amination. The reaction provides access to valuable chiral α-tertiary amines directly from an isomeric mixture of alkenes.
- Pan Xu
- , Jingjing Xie
- & X. Peter Zhang
-
Article
| Open Access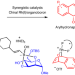 A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
Asymmetric systems for catalytic carbohydrate functionalization are mostly limited to chiral copper complexes and organocatalysts. Now, a synergistic chiral Rh(I)- and organoboron-catalysed site-selective functionalization of carbohydrate polyols has been developed, giving stereocontrolled access to biologically relevant arylhydronaphthalene glycosides. Enantio-, diastereo-, regio- and anomeric control and dynamic kinetic resolution were found to be concomitantly operative.
- V. U. Bhaskara Rao
- , Caiming Wang
- & Charles C. J. Loh
-
Article |
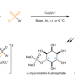 Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Heteroatom–heteroatom cross-couplings have so far remained elusive. Now, a copper-catalysed enantioselective S–O cross-coupling of diverse diols or triols with sulfonyl chlorides has been realized via a single-electron reductive elimination manifold. The reaction provides access to value-added chiral C3 building blocks and inositol phosphates through enantioselective desymmetrization of biomass-derived alcohols.
- Yong-Feng Cheng
- , Zhang-Long Yu
- & Xin-Yuan Liu
-
Article |
 Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
The enantioselective functionalization of C60 is highly challenging, typically requiring complex chiral tethers or demanding chromatography. Fullerenes have now been shown to undergo Diels–Alder reactions in a chemo-, regio- and enantio-selective fashion through confinement within an enantiopure metal–organic cage functionalized with a chiral formylpyridine group.
- Zifei Lu
- , Tanya K. Ronson
- & Jonathan R. Nitschke
-
News & Views |
 Radical arenes
Radical arenes
Radical-mediated functionalization streamlines access to complex synthetic targets. Now, a sulfonium-based donor–acceptor pair enables photoinduced charge-transfer interactions to access electronically diverse aryl radicals. Reaction with enol ethers or isocyanide provides a metal-free method for arene functionalization.
- Taylor N. Bednar
- & David A. Nagib
-
-
Article |
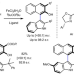 Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Direct oxidative methods for the enantioselective synthesis of heterobiaryl compounds that exhibit axial chirality remain elusive. Now, the use of an iron catalyst in the presence of a chiral PyBOX ligand and an oxidant enables the direct coupling of naphthols and indoles with high levels of enantio- and cross-selectivity.
- Richard R. Surgenor
- , Xiangqian Liu
- & Martin D. Smith
-
Article |
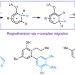 meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution
meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution
The strong regiochemical preferences of electrophilic aromatic substitution have played a key role in defining the diversity of accessible chemical space. Now, it has been shown that the electrophilic arylation of phenols can be achieved at the electronically disfavoured meta-position via a formal 1,2-migration of a key σ-complex intermediate.
- Aaron Senior
- , Katie Ruffell
- & Liam T. Ball
-
Article |
 A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation
A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation
Photoactivation of EDA complexes was previously limited to electronically biased partners to secure productive charge-transfer interactions. Now, the participation of triarylsulfonium salts—formed by selective C–H sulfenylation—in photoactive EDA complexes with catalytic triarylamine donors provides a site-selective and metal-free strategy for the generation of aryl radicals and the formal C–H functionalization of native arenes.
- Abhishek Dewanji
- , Leendert van Dalsen
- & David J. Procter
Browse broader subjects
Browse narrower subjects
- Asymmetric synthesis
- Automation
- Biomimetic synthesis
- Catalyst synthesis
- Chemical libraries
- Diversity-oriented synthesis
- Flow chemistry
- Fluorescent labelling
- Synthetic chemistry methodology
- Microwave chemistry
- Nanoparticle synthesis
- Natural product synthesis
- Polymer synthesis
- Reactive precursors
- Solid-phase synthesis

 Regioselective, catalytic 1,1-difluorination of enynes
Regioselective, catalytic 1,1-difluorination of enynes Rings of power
Rings of power Chemistry just a click away
Chemistry just a click away