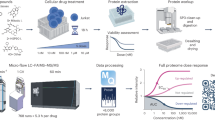
Abstract
Target identification involves deconvoluting the protein target of a pharmacologically active, small-molecule ligand, a process that is critical for early drug discovery yet technically challenging. Photoaffinity labelling strategies have become the benchmark for small-molecule target deconvolution, but covalent protein capture requires the use of high-energy ultraviolet light, which can complicate downstream target identification. Thus, there is a strong demand for alternative technologies that allow for controlled activation of chemical probes to covalently label their protein target. Here we introduce an electroaffinity labelling platform that leverages the use of a small, redox-active diazetidinone functional group to enable chemoproteomic-based target identification of pharmacophores within live cell environments. The underlying discovery to enable this platform is that the diazetidinone can be electrochemically oxidized to reveal a reactive intermediate useful for covalent modification of proteins. This work demonstrates the electrochemical platform to be a functional tool for drug-target identification.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the main findings of this work are available within the article and Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under the deposition number CCDC 2093827 (11). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Bunnage, M. E., Chekler, E. L. P. & Jones, L. H. Target validation using chemical probes. Nat. Chem. Biol. 9, 195–199 (2013).
Emmerich, C. H. et al. Improving target assessment in biomedical research: the GOT-IT recommendations. Nat. Rev. Drug Discov. 20, 64–81 (2021).
Kiriiri, G. K., Njogu, P. M. & Mwangi, A. N. Exploring different approaches to improve the success of drug discovery and development projects: a review. Future J. Pharm. Sci. 6, 27 (2020).
Bunnage, M. E. Getting pharmaceutical R&D back on target. Nat. Chem. Biol. 7, 335–339 (2011).
Schenone, M., Dančík, V., Wagner, B. K. & Clemons, P. A. Target identification and mechanism of action in chemical biology and drug discovery. Nat. Chem. Biol. 9, 232–240 (2013).
Park, J., Koh, M. & Park, S. B. From noncovalent to covalent bonds: a paradigm shift in target protein identification. Mol. Biosyst. 9, 544–550 (2012).
Sumranjit, J. & Chung, S. J. Recent advances in target characterization and identification by photoaffinity probes. Molecules 18, 10425–10451 (2013).
Smith, E. & Collins, I. Photoaffinity labeling in target- and binding-site identification. Future Med. Chem. 7, 159–183 (2015).
Li, Z. et al. Design and synthesis of minimalist terminal alkyne‐containing diazirine photo‐crosslinkers and their incorporation into kinase inhibitors for cell‐ and tissue‐based proteome profiling. Angew. Chem. Int. Ed. 52, 8551–8556 (2013).
Murale, D. P., Hong, S. C., Haque, Md. M. & Lee, J.-S. Photo-affinity labeling (PAL) in chemical proteomics: a handy tool to investigate protein–protein interactions (PPIs). Proteome Sci. 15, 14 (2017).
West, A. V. et al. Labeling preferences of diazirines with protein biomolecules. J. Am. Chem. Soc. 143, 6691–6700 (2021).
Conway, L. P. et al. Evaluation of fully-functionalized diazirine tags for chemical proteomic applications. Chem. Sci. 12, 7839–7847 (2021).
Brunner, J. New photolabeling and crosslinking methods. Annu. Rev. Biochem. 62, 483–514 (1993).
Kojetin, D. J. & Burris, T. P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 13, 197–216 (2014).
Uriz-Huarte, A. et al. The transcriptional repressor REV-ERB as a novel target for disease. Bioorg. Med. Chem. Lett. 30, 127395 (2020).
Solt, L. A. et al. Regulation of circadian behavior and metabolism by synthetic REV-ERB agonists. Nature 485, 62–68 (2021).
Mackay, A. S., Payne, R. J. & Malins, L. R. Electrochemistry for the chemoselective modification of peptides and proteins. J. Am. Chem. Soc. 144, 23–41 (2022).
Alvarez-Dorta, D. et al. Electrochemically promoted tyrosine-click-chemistry for protein labeling. J. Am. Chem. Soc. 140, 17120–17126 (2018).
Song, C. et al. Electrochemical oxidation induced selective tyrosine bioconjugation for the modification of biomolecules. Chem. Sci. 10, 7982–7987 (2019).
Toyama, E. et al. Electrochemical tryptophan-selective bioconjugation. Preprint at chemRxiv https://doi.org/10.26434/chemrxiv.7795484 (2019).
Stang, P. J. Unsaturated carbenes. Chem. Rev. 78, 383–405 (1978).
Minard, A., Liano, D., Wang, X. & Antonio, M. D. The unexplored potential of quinone methides in chemical biology. Bioorgan. Med. Chem. 27, 2298–2305 (2019).
Tomioka, H., Hayashi, N., Izawa, Y. & Liu, M. T. H. Photolysis of 3-chlorodiazirine in the presence of alkenes. Kinetic evidence for intervention of a carbene–alkene intermediate in addition of chlorocarbene to alkene. J. Am. Chem. Soc. 106, 454–456 (1984).
Voica, A.-F., Mendoza, A., Gutekunst, W. R., Fraga, J. O. & Baran, P. S. Guided desaturation of unactivated aliphatics. Nat. Chem. 4, 629–635 (2012).
Li, L., Li, Y., Fu, N., Zhang, L. & Luo, S. Catalytic asymmetric electrochemical α‐arylation of cyclic β‐ketocarbonyls with anodic benzyne intermediates. Angew. Chem. Int. Ed. 59, 14347–14351 (2020).
Funder, E. D., Trads, J. B. & Gothelf, K. V. Oxidative activation of dihydropyridine amides to reactive acyl donors. Org. Biomol. Chem. 13, 185–198 (2014).
Sterk, H., Uray, G. & Ziegler, E. Über Einen Versuch Zur Berechnung Der Fragmentation von β-Lactamen Mittels Der EHT-Methode. Monatsh. Chem. 103, 615–623 (1972).
Jungheim, L. N. in Advances in Heterocyclic Chemistry Vol. 110 (ed. Katritzky, A.) Ch. 5, 145–174 (Elsevier, 2013).
Zuhl, A. M. et al. Competitive activity-based protein profiling identifies aza-β-lactams as a versatile chemotype for serine hydrolase inhibition. J. Am. Chem. Soc. 134, 5068–5071 (2012).
Liu, C. & Szostak, M. Twisted amides: from obscurity to broadly useful transition‐metal‐catalyzed reactions by N−C amide bond activation. Chem. Eur. J. 23, 7157–7173 (2017).
Berlin, J. M. & Fu, G. C. Enantioselective nucleophilic catalysis: the synthesis of aza‐β‐lactams through [2+2] cycloadditions of ketenes with azo compounds. Angew. Chem. Int. Ed. 47, 7048–7050 (2008).
Tyler, D. S. et al. Click chemistry enables preclinical evaluation of targeted epigenetic therapies. Science 356, 1397–1401 (2017).
Jeong, S.-H., Jeon, Y.-J. & Park, S. J. Inhibitory effects of dieckol on hypoxia-induced epithelial-mesenchymal transition of HT29 human colorectal cancer cells. Mol. Med. Rep. 14, 5148–5154 (2016).
Yu, C., Wang, L., Zhu, Z., Bao, N. & Gu, H. Trans-membrane electron transfer in red blood cells immobilized in a chitosan film on a glassy carbon electrode. Microchim. Acta 181, 55–61 (2014).
Kumar, A. et al. The ins and outs of microorganism–electrode electron transfer reactions. Nat. Rev. Chem. 1, 0024 (2017).
Mohawk, J. A., Green, C. B. & Takahashi, J. S. Central and peripheral circadian clocks in mammals. Neuroscience 35, 445–462 (2012).
Pariollaud, M. et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J. Clin. Invest. 128, 2281–2296 (2018).
Woldt, E. et al. Rev-Erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 19, 1039–1046 (2013).
Geldof, L., Deventer, K., Roels, K., Tudela, E. & Eenoo, P. V. In vitro metabolic studies of REV-ERB agonists SR9009 and SR9011. Int. J. Mol. Sci. 17, 1676 (2016).
Chang, C. et al. The nuclear receptor REV-ERBα modulates Th17 cell-mediated autoimmune disease. Proc. Natl Acad. Sci. USA 116, 18528–18536 (2019).
Sulli, G. et al. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene induced senescence. Nature 553, 351–355 (2018).
Dierickx, P. et al. SR9009 has REV-ERB–independent effects on cell proliferation and metabolism. Proc. Natl Acad. Sci. USA 116, 12147–12152 (2019).
Acknowledgements
The work was supported by the National Science Foundation Center for Synthetic Organic Electrochemistry CHE-2002158 (exploration and development of electrochemically active functional group), National Institutes of Health grant GM-118176 (synthesis of elaborated probes for biological studies) and gifts from Merck & Co., Inc., Kenilworth, NJ, USA (synthesis of elaborated probes for biological studies). G.N.H. was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), grant numbers 419055018/HE 8427/1-1. A.F.S. was supported by the Lundbeck Foundation (grant number R208-2015-3354), E.R.-C. was supported by the Galician Programme for Research, Innovation and Growth for 2018. We thank D.-H. Huang and L. Pasternack (Scripps Research) for assistance with NMR spectroscopy; and J. Chen, B. Sanchez and E. Sturgell (Automated Synthesis Facility, Scripps Research) for purification of compounds and acquisition of HRMS data. We thank T. Wyche (Merck & Co., Inc.) for assistance with HRMS data, S. Ingale (Merck & Co., Inc.) for assistance with peptide synthesis, and J. Oh (Merck & Co., Inc.) for helpful discussions.
Author information
Authors and Affiliations
Contributions
R.C.O., O.O.F. and P.S.B. conceptualized the study. Y.K., G.N.H., A.F.S., J.C.V., E.R.-C. and P.S.B. designed and performed chemical experiments. K.A.R., L.A.A., A.K.O., L.R.R., R.C.O. and O.O.F. designed and performed biological experiments. All the authors contributed to data analysis. Y.K., K.A.R., R.C.O., O.O.F. and P.S.B. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
K.A.R., L.A.A., A.K.O., L.R.R., R.C.O., and O.O.F. are/were employees of Merck and Co., Inc. during the preparation of this manuscript.
Peer review
Peer review information
Nature Chemistry thanks Sébastien Gouin, Christina Woo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Nature chemistry Supplementary Information final.
Supplementary Information
Supplementary data table.
Supplementary Data 1
Source data for Supplementary Information.
Supplementary Data 2
Unprocessed western blots for Supplementary data.
Supplementary Data 3
Microscopy images for Supplementary data.
Supplementary Data 4
X-ray crystal structure CIF file.
Source data
Source Data Fig. 3
Unprocessed western blot.
Source Data Fig. 3
Peptide mapping spectral count.
Source Data Fig. 4
Gene Ontology data.
Source Data Fig. 5
Cell assay data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawamata, Y., Ryu, K.A., Hermann, G.N. et al. An electroaffinity labelling platform for chemoproteomic-based target identification. Nat. Chem. 15, 1267–1275 (2023). https://doi.org/10.1038/s41557-023-01240-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01240-y
This article is cited by
-
Photoaffinity labelling with small molecules
Nature Reviews Methods Primers (2024)