Abstract
The ortho-substituted phenyl ring is a basic structural element in chemistry. It is found in more than three hundred drugs and agrochemicals. During the past decade, scientists have tried to replace the phenyl ring in bioactive compounds with saturated bioisosteres to obtain novel patentable structures. However, most of the research in this area has been devoted to the replacement of the para-substituted phenyl ring. Here we have developed saturated bioisosteres of the ortho-substituted phenyl ring with improved physicochemical properties: 2-oxabicyclo[2.1.1]hexanes. Crystallographic analysis revealed that these structures and the ortho-substituted phenyl ring indeed have similar geometric properties. Replacement of the phenyl ring in marketed agrochemicals fluxapyroxad (BASF) and boscalid (BASF) with 2-oxabicyclo[2.1.1]hexanes dramatically improved their water solubility, reduced lipophilicity and most importantly retained bioactivity. This work suggests an opportunity for chemists to replace the ortho-substituted phenyl ring in bioactive compounds with saturated bioisosteres in medicinal chemistry and agrochemistry.

Similar content being viewed by others
Main
The phenyl ring is a basic structural element in chemistry. Moreover, it is one of the most common rings in bioactive compounds1. However, organic compounds with more than two phenyl rings often have poor solubility and low metabolic stability—undesired effects in medicinal chemistry2. In this context, during the last decade, the concept ‘escape from flatland’ became popular3,4. Today, medicinal chemists prefer using F(sp3)-rich structures in drug discovery projects5,6,7,8,9,10. The replacement of the phenyl ring in bioactive compounds with saturated bioisosteres has become a popular tactic to obtain novel structures with an improved physicochemical profile11,12,13,14. However, most of the research in this area is devoted to the replacement of monosubstituted and para-disubstituted phenyl rings12,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49.
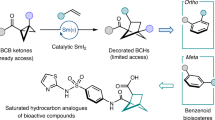
Ortho-disubstituted phenyl rings are found in more than three hundred drugs and agrochemicals (www.drugbank.ca; Fig. 1a) (ref. 50). For example, aspirin, which is widely known, contains an ortho-disubstituted phenyl ring in its structure. In 2008, the first example of mimicking an ortho-disubstituted phenyl ring in a bioactive compound with a saturated bioisostere, cyclopropane, appeared in the literature (Fig. 1b) (ref. 51). Later, similar studies were performed with 1,2-disubstituted cyclopentanes and cyclohexanes (Fig. 1b) (ref. 52). In the past two years, great progress has been achieved with saturated bicyclic scaffolds, which, compared to previously used monocyclic counterparts, are intrinsically conformationally rigid. In particular, 1,2-disubstituted bicyclo[1.1.1]pentanes (Fig. 1c) (refs. 53,54) and bicyclo[2.1.1]hexanes (Fig. 1c) (refs. 55,56,57,58,59,60,61) were used as saturated bioisosteres of the ortho-disubstituted phenyl ring. In this work, we report on the preparation, characterization and biological validation of the next generation of these saturated bioisosteres, 2-oxabicyclo[2.1.1]hexanes, analogues of the ortho-substituted phenyl ring with improved physicochemical properties (Fig. 1c).
a, The ortho-substituted phenyl ring is a part of >300 drugs and agrochemicals. b, Previous examples of replacement of the ortho-substituted phenyl ring in bioactive compounds with monocyclic saturated rings by Qiao (2008) (ref. 51) and Shinozuka (2020) (ref. 52). c, Previous examples of replacement of the ortho-substituted phenyl ring in bioactive compounds with bicyclic saturated scaffolds: bicyclo[1.1.1]pentane (Ma, 2020 (ref. 53); Baran, 2021 (ref. 54)) and bicyclo[2.1.1]hexane (Mykhailiuk, 2020 (ref. 55); Brown, 2022 (ref. 56); Procter, 2023 (ref. 57)). The aim of this work is the replacement of the ortho-substituted phenyl ring in bioactive compounds with 2-oxabicyclo[2.1.1]hexane.[+O], replacement of a methylene group \((-{\mathrm{CH}}_{2^-})\) with an oxygen atom (−O−).
Design
In the design of a core with a similar structure to bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes, but having reduced lipophilicity and enhanced water solubility, we decided to insert an oxygen atom. Replacing the methylene group in bicyclo[1.1.1]pentane with the oxygen atom leads to a strained oxetane structure (Fig. 1c), which is interesting but could be labile due to possible ring opening with nucleophiles62. Analogous replacement in bicyclo[2.1.1]hexanes, however, gives the substituted tetrahydrofuran (Fig. 1c). That core should be more chemically stable, so we decided to make it. From a medicinal chemistry perspective, having the ether oxygen atom in the molecule is also useful, since it could serve as an additional binding site to a receptor.
Inspiration came from our previous work, where we synthesized bridgehead disubstituted 2-oxabicyclo[2.1.1]hexanes and believed they could mimic the meta-disubstituted phenyl ring in bioactive compounds63. This hypothesis, however, was not validated. Therefore, here we decided to prepare the disubstituted 2-oxabicyclo[2.1.1]hexanes and biologically validate them as bioisosteres of the ortho-disubstituted phenyl ring.
Synthesis
The photochemical [2 + 2] cycloaddition between alkenes proved to be a powerful strategy to construct cyclobutanes64. In this context, we wondered if diene 1 (easily obtained from the commercially available starting materials; Fig. 2a) would undergo an intramolecular cyclization into the needed 2-oxabicyclo[2.1.1]hexane core. Direct irradiation of diene 1 in acetonitrile under different wavelengths gave only traces of product (Table 1, entries 1–4). Irradiation with a Hanovia broad wavelength mercury lamp gave the needed product along with many side products (entry 5). Next, we tried the addition of available organic ketones for the triplet sensitization of the styrene moiety. Indeed, smooth formation of the needed product 1a (d.r. = ~4:1) was observed under irradiation at 368 nm. The best result was obtained with benzophenone (entry 7), whereas acetophenone and substituted benzophenones also worked but provided lower yields of the end product (entries 6, 8 and 9). Among all tested solvents (entries 10–13), the best outcome was obtained in acetonitrile. Without irradiation, the reaction did not take place at room temperature or with heating (entries 14 and 15).
a, Gram-scale synthesis of compound 1b. THF, tetrahydrofuran; quant., quantitative. b, X-ray crystal structures of compounds 5b and 9b. Hydrogen atoms are omitted for clarity. Carbon, grey; oxygen, red; fluorine, green. c, Definition of vectors n1 and n2, and geometric parameters d, r, φ1, φ2 and θ. Ortho-substituted phenyl ring and 2-oxabicyclo[2.1.1]hexane are shown as examples. d, Geometric parameters d, r, φ1, φ2 and |θ| for ortho-substituted benzenes (valsartan, telmisartan), the saturated literature bioisosteres 25–27 and water-soluble saturated bioisosteres 5b and 9b. aData is taken from ref. 67. bData is taken from ref. 68. cData is taken from ref. 54. dData is taken from ref. 55.
Under optimized conditions, cyclization of diene 1 led to a rather clean formation of a diastereomeric mixture of products 1a (d.r. = 4:1); however, the pure major isomer 1a was isolated by column chromatography in only 56% yield. The separation of isomers was problematic and led to a notable loss of yield, which needed to be solved.
Scaled-up synthesis
The optimized synthetic protocol is shown in Fig. 2a. It was important to identify a method that employed only available and cheap starting materials. The synthesis started from propargyl alcohol (2). Copper-catalysed reaction with phenyl magnesium bromide gave alcohol 3 in 71% yield following the reported procedure65. A Michael addition of the latter with methyl propiolate (4) in the presence of (1,4-diazabicyclo[2.2.2]octane) (DABCO) provided the needed diene 1. We mentioned that compound 1 partially decomposed during column chromatography and under storage at room temperature. Therefore, we decided to generate crude diene 1 in situ and use it directly in the photochemical step (Supplementary Information, page 6). A mixture of isomers 1a was obtained. After extensive experimentation, we found a way to avoid column chromatography and not lose the yield. The crude reaction mixture after irradiation (isomers 1a and benzophenone) was saponified with sodium hydroxide. A standard workup (removal of benzophenone) followed by crystallization from a hexane–MeOtBu mixture to remove the minor isomer allowed the isolation of pure major isomer 1b at 71% yield in three steps from alcohol 3. Product 1b was obtained on a ten gram scale with no column purifications.
Scope
Next, we studied the scope of the developed method. The photocyclization method tolerated various substituents on the aromatic core (Table 2). Among them were alkyl groups (5a–8a), fluorine atoms (9a–11a) and chlorine atoms (12a and 13a), methoxy groups (14a–16a) and trifluoromethyl groups (17a–19a). The reaction was also compatible with various substituted pyridines (20a–24a). In all cases, we isolated analytical quantities of intermediate esters 5a–24a by column chromatography to characterize them. On a gram scale, however, we directly used crude reaction mixtures with 5a–24a after photocyclization in the subsequent saponification step. In half of all cases, we could obtain the final carboxylic acids by simple crystallization of crude reaction mixtures from various solvents (Table 2). In the other half of the cases, column chromatography was still needed. The structure of carboxylic acids 5b and 9b was confirmed by X-ray crystallographic analysis (Fig. 2b).
Chemical stability
We also checked the chemical stability of three representative carboxylic acids, 1b, 19b and 22b (Table 2), because we suspected that some of them could decompose via a retro-Michael-type reaction. Treatment of them with aqueous 1 M hydrochloric acid or aqueous 1 M sodium hydroxide at room temperature for one day did not lead to any decomposition. All products were crystalline solids, and we stored all of them in closed vials at room temperature on the shelf. The 1H-NMR, liquid chromatography–mass spectrometry (LC-MS) inspection after three months did not reveal any decomposition.
Crystallographic analysis
Next, we compared the geometric parameters of 2-oxabicyclo[2.1.1]hexanes with those of the ortho-substituted phenyl ring and their previously suggested saturated bioisosteres, bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. For that, we employed the exit vector plots tool66. In this method, substituents at the disubstituted scaffold were simulated by two exit vectors n1 and n2 (Fig. 2c). The relative spatial arrangement of vectors is described by four geometric parameters: the distance between C-C atoms r; the plane angles φ1 (between n1 and the C atom) and φ2 (between n2 and the C atom); and the dihedral angle θ defined by vectors n1, C-C and n2. An additional important parameter—the distance d between two carbon substituents (Fig. 2c)—was also measured.
We calculated the values of d, r, φ1, φ2 and θ of 2-oxabicyclo[2.1.1]hexanes from the X-ray data of compounds 5b and 9b. The related parameters for bicyclo[1.1.1]pentane 25 (ref. 54) and bicyclo[2.1.1]hexanes 26 and 27 (ref. 55) were calculated from their X-ray data published in the literature. The corresponding parameters for ortho-substituted phenyl rings were calculated from the reported crystal data of two antihypertensive drugs—valsartan67 and telmisartan68 (Fig. 2d). Analysis of this data revealed that the geometric properties of 2-oxabicyclo[2.1.1]hexanes in general were similar to those of the ortho-substituted phenyl ring. In particular, the distance r in 2-oxabicyclo[2.1.1]hexanes was ~0.2 Å longer than that in the ortho-phenyl ring: 1.56–1.57 Å versus 1.38–1.44 Å, respectively. The distance d between substituents in 2-oxabicyclo[2.1.1]hexanes was also ~0.5 Å longer than that in the ortho-phenyl ring: 3.6 Å versus 3.0–3.1 Å, respectively. Angles φ1 and φ2 were almost identical in both scaffolds. Moreover, φ1 and φ2 in 2-oxabicyclo[2.1.1]hexanes were much closer to those in the ortho-phenyl ring, than to those of the previously used saturated bioisosteres: bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. The difference in planarity was notable: while ortho-phenyl was almost flattened (|θ| = 7–8°), 2-oxabicyclo[2.1.1]hexanes had a substantial three-dimensional character: |θ| = 80°. It must be noted, however, that non-planarity was also present in bicyclo[1.1.1]pentanes (|θ| = 58°) and bicyclo[2.1.1]hexanes (|θ| = ~75°; Fig. 2d).
In general, vector characteristics of 2-oxabicyclo[2.1.1]hexanes were very similar to those of the previously used bioisosteres of the ortho-substituted phenyl ring: bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes. Moreover, the important angles φ1 and φ2 in 2-oxabicyclo[2.1.1]hexanes were even closer to those in the ortho-phenyl ring than to those in bicyclo[1.1.1]pentanes and bicyclo[2.1.1]hexanes.
Incorporation into bioactive compounds
The incorporation of the 2-oxabicyclo[2.1.1]hexane scaffold into bioactive compounds was attempted next. We chose four bioactive products with the ortho-substituted phenyl ring: agrochemical fungicides fluxapyroxad and boscalid, antibacterial agent phthalylsulfathiazole and lipid-lowering agent lomitapide (Fig. 3).
a, Synthesis and properties of compounds 28 and 29, saturated bioisosteres of fluxapyroxad. Δ, heating; Het-CO2H, 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid. b, Synthesis and properties of compounds 30 and 31, saturated bioisosteres of boscalid. Het-CO2H, 2-chloropyridine-3-carboxylic acid. c, Synthesis and properties of compounds 32 and 33, saturated bioisosteres of phthalylsulfathiazole. DIPEA, N,N-diisopropylethylamine; DMF, dimethylformamide. d, Synthesis and properties of compounds 34 and 35, saturated bioisosteres of Lomitapide. Solubility (in µM) refers to the experimental kinetic solubility in phosphate-buffered saline at pH 7.4; clogP is the calculated lipophilicity; logD (7.4) refers to the experimental distribution coefficient in n-octanol/phosphate-buffered saline at pH 7.4. Reliable logD measures were obtained within a range 1.0–4.5. CIint, intrinsic clearance, experimental metabolic stability in human liver microsomes (in µl min–1 mg–1). cat., catalyst.
Synthesis of the saturated analogue of fluxapyroxad was undertaken from carboxylic acid 11b (Fig. 3a). The standard Curtius reaction followed by acylation of the intermediate amine with the substituted pyrazole carboxylic acid gave the needed compound 29. Using an analogous tactic, compound 31—a saturated analogue of boscalid—was also obtained from carboxylic acid 17b (Fig. 3b). The saturated analogue of phthalylsulfathiazole was obtained by converting carboxylic acid 16b first into the methyl ester followed by the oxidation of the phenyl ring. Amide coupling of the formed acid with the para-substituted aniline followed by saponification of the methyl ester gave the final compound 33 (Fig. 3c). Amide coupling of carboxylic acid 19b with the correspondingly N-substituted 4-aminopiperidine gave compound 35, a saturated analogue of lomitapide (Fig. 3d).
In all cases, in addition to bioactive compounds with a 2-oxabicyclo[2.1.1]hexane core (29, 31, 33 and 35), we also synthesized analogous carbocyclic analogues 28, 30, 32 and 34 (Fig. 3 and Supplementary Information, pages 34–42).
Physicochemical parameters
In the next step, we studied the effect of the replacement of the ortho-phenyl ring by 2-oxabicyclo[2.1.1]hexanes on the physicochemical properties of bioactive compounds. For the comparison, we also used the corresponding carbocyclic core, bicyclo[2.1.1]hexane.
Water solubility
Replacement of the ortho-substituted phenyl ring in fluxapyroxad by bicyclo[2.1.1]hexane (28) slightly increased its solubility (Fig. 3a). However, incorporation of the 2-oxabicyclo[2.1.1]hexane in fluxapyroxad (29) resulted in a dramatic sixfold increase in solubility: 25 µM (fluxapyroxad) versus 34 µM (28) versus 155 µM (29). An analogous trend was also seen with boscalid and its analogues 30 and 31 (Fig. 3b). Replacement of the phenyl ring in boscalid with bicyclo[2.1.1]hexane (30) led to the increase of solubility by ~50%. However, the corresponding replacement with 2-oxabicyclo[2.1.1]hexane (31) increased the solubility by more than ten times: 11 µM (boscalid) versus 17 µM (30) versus 152 µM (31). Replacement of the phenyl ring in phthalylsulfathiazole with bicyclo[2.1.1]hexane (32) decreased its solubility, while the incorporation of the 2-oxabicyclo[2.1.1]hexane core (33) restored it: 170 µM (phthalylsulfathiazole) versus 101 µM (32) versus 158 µM (33; Fig. 3c). Lomitapide had poor solubility in water, and replacement of the phenyl ring in lomitapide with saturated bioisosteres (34 and 35) did not have any substantial impact on the solubility (Fig. 3d).
In summary, in two (fluxapyroxad, boscalid) out of four bioactive compounds, replacement of the ortho-substituted phenyl ring with 2-oxabicyclo[2.1.1]hexane led to a dramatic increase in water solubility by about one order of a magnitude.
Lipophilicity
To estimate the influence of the replacement of the ortho-substituted phenyl ring with saturated bioisosteres on lipophilicity, we used two parameters: calculated lipophilicity, log P, where P is the partition coefficient (clogP), and experimental lipophilicity, log D, where D is the distribution coefficient (logD).
Replacement of the phenyl ring with bicyclo[2.1.1]hexane either led to an increase of clogP (fluxapyroxad, boscalid; Fig. 3a,b) or did not affect it (phthalylsulfathiazole, lomitapide; Fig. 3c,d). However, in all four bioactive compounds, incorporation of 2-oxabicyclo[2.1.1]hexane instead of the ortho-substituted phenyl ring led to a decrease of the clogP index by about one unit.
The effect of the replacement of the ortho-substituted phenyl ring with saturated bioisosteres on the logD index was more complex. In fluxapyroxad, incorporation of the bicyclo[2.1.1]hexane core increased logD, while the incorporation of 2-oxabicyclo[2.1.1]hexane slightly decreased it: 3.5 (fluxapyroxad) versus 4.3 (28) versus 2.8 (29; Fig. 3a). In boscalid, the incorporation of the bicyclo[2.1.1]hexane core did not affect logD substantially, while the incorporation of 2-oxabicyclo[2.1.1]hexane reduced it: 3.6 (boscalid) versus 3.5 (28) versus 2.7 (29; Fig. 3b).
In summary, in all tested compounds, replacement of the ortho-substituted phenyl ring with 2-oxabicyclo[2.1.1]hexane decreased the lipophilicity as measured by both clogP and logD indexes by 0.5–1.4 units.
Metabolic stability
The effect of saturated bioisosteres on the metabolic stability of bioactive compounds was complex and depended on the chemical structure. In fluxapyroxad, the incorporation of bicyclo[2.1.1]hexane (28) decreased the metabolic stability (Fig. 3a). However, incorporation of 2-oxabicyclo[2.1.1]hexane (29) unexpectedly increased it: the experimental metabolic stability in human liver microsomes, intrinsic clearance, CIint (mg min–1 μl–1) = 28 (fluxapyroxad) versus 35 (28) versus 23 (29). In boscalid, incorporation of the bicyclo[2.1.1]hexane (30) increased the metabolic stability, but the incorporation of 2-oxabicyclo[2.1.1]hexane (31) increased it even more: CIint (mg min–1 μl–1) = 26 (boscalid) versus 12 (30) versus 3 (31; Fig. 3b). All three compounds, phthalylsulfathiazole and its two saturated analogues 32 and 33, were metabolically stable (Fig. 3c). In lomitapide, incorporation of the bicyclo[2.1.1]hexane core (34) decreased the metabolic stability, but the incorporation of the 2-oxabicyclo[2.1.1]hexane core (35) somewhat restored it: CIint (mg min–1 μl–1) = 55 (lomitapide) versus 157 (34) versus 87 (35; Fig. 3d).
In summary, replacement of the ortho-substituted phenyl ring with 2-oxabicyclo[2.1.1]hexane in bioactive compounds improved metabolic stability (CIint) in boscalid and fluxapyroxad; slightly decreased it in lomitapide; and did not affect it in phthalylsulfathiazole.
Bioactivity
Finally, we wanted to answer a key question: can 2-oxabicyclo[2.1.1]hexanes indeed mimic the ortho-substituted phenyl ring in real-world bioactive compounds? Fluxapyroxad and boscalid are marketed fungicides, developed by BASF, that have been approved for use in the United States and the European Union. Therefore, we measured their antifungal activity and compared it to that of their saturated analogues 28–31. In strict contrast to medicinal chemistry, the use of racemic mixtures in agrochemistry is common50; therefore for the primary validation of the proof-of-concept, we directly studied the biological activity of the available racemic compounds 28–31 (Fig. 4).
a,b, Inhibition of growth of F. oxysporum (a) and F. verticillioides (b; measured as a diameter d of the inhibition zone, in millimetres), by fluxapyroxad and its saturated analogues 28 and 29 at different concentrations after 48 h of incubation. c,d, Inhibition of growth of F. oxysporum (c) and F. verticillioides (d; measured as a diameter of the inhibition zone, in millimetres) by boscalid and its saturated analogues 30 and 31 at different concentrations after 48 h of incubation. e, MIC for fluxapyroxad and its analogues 28 and 29, and for boscalid and its analogues 30 and 31. aMaximal inhibition of growth of F. oxysporum by 38.0 ± 1.9% at a concentration of 0.031 mg ml–1. bMaximal inhibition of growth of F. oxysporum by 35.7 ± 2.4% at a concentration of 0.125 mg ml–1. cMaximal inhibition of growth of F. verticillioides by 39.2 ± 2.7% at a concentration of 0.063 mg ml–1. dMaximal inhibition of growth of F. verticillioides 36.3 ± 1.9% at a concentration of 0.250 mg ml–1.
First, we measured the antifungal activity of all compounds using the agar well diffusion method (Supplementary Information, pages 238–243). Fluxapyroxad, and its saturated analogues 28 and 29, showed a similar trend in activity at the inhibition of fungi growth (Fig. 4a,b). The 2-oxabicyclo[2.1.1]hexane analogue 29 was active, but less potent compared to the original fungicide. Compound 29 and fluxapyroxad almost identically inhibited the growth of Fusarium oxysporum at high concentrations; however, at low concentrations, analogue 29 showed lower activity (Fig. 4a). Similarly, 29 and fluxapyroxad effectively inhibited the growth of Fusarium verticillioides at high concentrations; however, at low concentrations, only fluxapyroxad remained active, while analogue 29 did not (Fig. 4b).
Boscalid and both saturated analogues 30 and 31 also effectively inhibited the fungi growth (Fig. 4c,d). However, 2-oxabicyclo[2.1.1]hexane 31 was slightly less potent than boscalid at the inhibition of F. oxysporum (Fig. 4c) and notably less potent at the inhibition of F. verticillioides, especially at low concentrations (Fig. 4d).
Additionally, we measured a minimal inhibitory concentration (MIC) of all compounds (Fig. 4e). Interestingly, fluxapyroxad and its 2-oxabicyclo[2.1.1]hexane analogue 29 exhibited equal MIC values of 0.250 mg ml–1 at the inhibition of the growth of F. verticillioides. Carbocyclic analogue 30 was two times as potent: MIC = 0.125 mg ml–1. At the same time, boscalid and its 2-oxabicyclo[2.1.1]hexane analogue 31 also exhibited equal MIC values of 0.250 mg ml–1 at the inhibition of the growth of F. oxysporum. Carbocyclic analogue 30 was much more potent: MIC = 0.031 mg ml–1.
Conclusion
The ortho-substituted phenyl ring (as well as meta and para isomers) is a basic structural element in chemistry. In this work, we synthesized, characterized and studied 2-oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring (Fig. 1c). These scaffolds were synthesized from readily available starting materials on a multigram scale. Crystallographic analysis revealed that these structures and the ortho-substituted phenyl ring indeed have similar geometric properties. Replacement of the ortho-substituted phenyl ring in bioactive compounds with 2-oxabicyclo[2.1.1]hexanes, in two out of four cases, dramatically improved water solubility (up to more than ten times) and metabolic stability. Moreover, in all four cases, such replacement also reduced lipophilicity by 0.5–1.4 clogP or logD units (Fig. 3b). In addition, the 2-oxabicyclo[2.1.1]hexanes 29 and 31 showed a similar antifungal activity compared to that of the original fungicides fluxapyroxad and boscalid.
Given the commonplace nature of the ortho-substituted phenyl ring in chemistry, we believe that its saturated bioisosteres described in this work will soon become very popular. One must always keep in mind, however, that the replacement of the phenyl ring in bioactive compounds with saturated isosteres can fail, if the phenyl ring is involved in integrations with the receptor: π–π stacking, π–amide stacking, π–Asp/Glu/Arg stacking, π to amide N–H, π to O–H, π to S–H, π to ammonium salts and so on. Therefore, the replacement of the phenyl ring in bioactive compounds with saturated isosteres must be careful and balanced69.
Methods
General procedure for the photochemical [2 + 2] cycloaddition
The solution of diene 1 (16.79 g, 0.077 mol, 1.0 equiv.) and benzophenone (1.40 g, 0.0077 mol, 0.10 equiv.) in 850 ml of dry CH3CN (concentration = 0.091 M) was put into a standard chemical 1 l glass flask. The reaction mixture was degassed by the bubbling of argon for 15 min. The flask was closed by a septum and irradiated with luminescent UV lamps at 368 nm (24 lamps; Sylvania 368 Blacklight F25/T8/18/BL3368; each lamp has nominal power of 25 W; total power is 600 W), under stirring at room temperature for 48 h. The reaction mixture was concentrated under reduced pressure to provide the crude product 1a that was used in the next step (saponification) without any purification.
NMR spectra were analysed with MestreNova (11.0.3-18688).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
Experimental data as well as characterization data for all new compounds prepared during these studies are provided in the Supplementary Information of this manuscript. The X-ray crystallographic coordinates for compounds 5b and 9b have been deposited at the Cambridge Crystallographic Data Center (CCDC) with accession codes 2166325 (5b) and 2166326 (9b). These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/structures/.
References
Taylor, R. D., MacCoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Ritchie, T. J. & Macdonald, S. J. F. The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design? Drug Discovery Today 14, 1011–1020 (2009).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. Med. Chem. Commun. 4, 515–519 (2013).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Dhake, K. et al. Beyond bioisosteres: divergent synthesis of azabicyclohexanes and cyclobutenyl amines from bicyclobutanes. Angew. Chem. Int. Ed. 61, e202204719 (2022).
Stepan, A. F., Kauffman, G. W., Keefer, C. E., Verhoest, P. R. & Edwards, M. Evaluating the differences in cycloalkyl ether metabolism using the design parameter “lipophilic metabolism efficiency” (LipMetE) and a matched molecular pairs analysis. J. Med. Chem. 56, 6985–6990 (2013).
Stepan, A. F. et al. Metabolism-directed design of oxetane-containing arylsulfonamide derivatives as γ-secretase inhibitors. J. Med. Chem. 54, 7772–7783 (2011).
Yang, Y. et al. Practical and modular construction of C(sp3)-rich alkyl boron compounds. J. Am. Chem. Soc. 143, 471–480 (2021).
Simlandy, A. K., Lyu, M.-Y. & Brown, M. K. Catalytic arylboration of spirocyclic cyclobutenes: rapid access to highly substituted spiro[3.n]alkanes. ACS Catal. 11, 12815–12820 (2021).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Locke, G. M., Bernhard, S. S. R. & Senge, M. O. Nonconjugated hydrocarbons as rigid-linear motifs: isosteres for material sciences and bioorganic and medicinal chemistry. Chem. Eur. J. 25, 4590–4647 (2019).
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Gianatassio, R. et al. Strain release amination. Science 351, 241–246 (2016).
Kanazawa, J., Maeda, K. & Uchiyama, M. Radical multicomponent carboamination of [1.1.1]propellane. J. Am. Chem. Soc. 139, 17791–17794 (2017).
Makarov, I. S., Brocklehurst, C. E., Karaghiosoff, K., Koch, G. & Knochel, P. Synthesis of bicyclo[1.1.1]pentane bioisosteres of internal alkynes and para-disubstituted benzenes from [1.1.1]propellane. Angew. Chem. Int. Ed. 56, 12774–12777 (2017).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope, and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Caputo, D. F. J. et al. Synthesis and applications of highly functionalized 1-halo-3-substituted bicyclo[1.1.1]pentanes. Chem. Sci. 9, 5295–5390 (2018).
Shelp, R. A. & Walsh, P. J. Synthesis of BCP benzylamines from 2-azaallyl anions and [1.1.1]propellane. Angew. Chem. Int. Ed. 57, 15857–15861 (2018).
Hughes, J. M. E., Scarlata, D. A., Chen, A. C.-Y., Burch, J. D. & Gleason, J. L. Aminoalkylation of [1.1.1]propellane enables direct access to high-value 3-alkylbicyclo[1.1.1]pentan-1-amines. Org. Lett. 21, 6800–6804 (2019).
Wong, M. L. J., Mousseau, J. J., Mansfield, S. J. & Anderson, E. A. Synthesis of enantioenriched α-chiral bicyclo[1.1.1]pentanes. Org. Lett. 21, 2408–2411 (2019).
Nugent, J. et al. A general route to bicyclo[1.1.1]pentanes through photoredox catalysis. ACS Catal. 9, 9568–9574 (2019).
Nugent, J. et al. Synthesis of all-carbon disubstituted bicyclo[1.1.1]pentanes by iron-catalyzed Kumada cross-coupling. Angew. Chem. Int. Ed. 59, 11866–11870 (2020).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Garlets, Z. J. et al. Enantioselective C–H functionalization of bicyclo[1.1.1]pentanes. Nat. Catal. 3, 351–357 (2020).
Schwärzer, K., Zipse, H., Karaghiosoff, K. & Knochel, P. Highly regioselective addition of allylic zinc halides and various zinc enolates to [1.1.1]propellane. Angew. Chem. Int. Ed. 59, 20235–20241 (2020).
Kim, J. H., Ruffoni, A., Al-Faiyz, Y. S. S., Sheikh, N. S. & Leonori, D. Divergent strain-release amino-functionalization of [1.1.1]propellane with electrophilic nitrogen-radicals. Angew. Chem. Int. Ed. 59, 8225–8231 (2020).
Yu, S., Jing, Dr. C., Noble, A. & Aggarwal, V. K. 1,3-Difunctionalizations of [1.1.1]propellane via 1,2-metallate rearrangements of boronate complexes. Angew. Chem. Int. Ed. 59, 3917–3921 (2020).
Yu, S., Jing, C., Noble, A. & Aggarwal, V. K. Iridium-catalyzed enantioselective synthesis of α-chiral bicyclo[1.1.1]pentanes by 1,3-difunctionalization of [1.1.1]propellane. Org. Lett. 22, 5650–5655 (2020).
Shelp, R. A. et al. Strain-release 2-azaallyl anion addition/borylation of [1.1.1]propellane: synthesis and functionalization of benzylamine bicyclo[1.1.1]pentyl boronates. Chem. Sci. 12, 7066–7072 (2021).
Wong, M. L. J., Sterling, A. J., Mousseau, J. J., Duarte, F. & Anderson, E. A. Direct catalytic asymmetric synthesis of α-chiral bicyclo[1.1.1]pentanes. Nat. Commun. 12, 1644 (2021).
Shin, S., Lee, S., Choi, W., Kim, N. & Hong, S. Visible-light-induced 1,3-aminopyridylation of [1.1.1]propellane with N-aminopyridinium salts. Angew. Chem. Int. Ed. 60, 7873–7879 (2021).
Pickford, H. D. et al. Twofold radical-based synthesis of N,C-difunctionalized bicyclo[1.1.1]pentanes. J. Am. Chem. Soc. 143, 9729–9736 (2021).
Nugent, J., Sterling, A. J., Frank, N., Mousseau, J. J. & Anderson, E. A. Synthesis of α-quaternary bicyclo[1.1.1]pentanes through synergistic organophotoredox and hydrogen atom transfer catalysis. Org. Lett. 23, 8628–8633 (2021).
Shelp, R. et al. Enantioenriched BCP benzylamine synthesis via metal hydride hydrogen atom transfer/sulfinimine addition to [1.1.1]propellane. Org. Lett. 24, 110–114 (2022).
Mousseau, J. J. et al. Automated nanomole-scale reaction screening toward benzoate bioisosteres: a photocatalyzed approach to highly elaborated bicyclo[1.1.1]pentanes. ACS Catal. 12, 600–606 (2022).
Livesley, S. et al. Electrophilic activation of [1.1.1]propellane for the synthesis of nitrogen-substituted bicyclo[1.1.1]pentanes. Angew. Chem. Int. Ed. 61, e202111291 (2022).
Polites, V. C., Badir, S. O., Keess, S., Jolit, A. & Molander, G. A. Nickel-catalyzed decarboxylative cross-coupling of bicyclo[1.1.1]pentyl radicals enabled by electron donor–acceptor complex photoactivation. Org. Lett. 23, 4828–4833 (2021).
Huang, W., Keess, S. & Molander, G. A. Dicarbofunctionalization of [1.1.1]propellane enabled by nickel/photoredox dual catalysis: one-step multicomponent strategy for the synthesis of BCP-aryl derivatives. J. Am. Chem. Soc. 144, 12961–12969 (2022).
Yen-Pon, E. et al. On-DNA hydroalkylation to introduce diverse bicyclo[1.1.1]pentanes and abundant alkyls via halogen atom transfer. J. Am. Chem. Soc. 144, 12184–12191 (2022).
Dong, W. et al. Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane. Nat. Chem. 14, 1068–1077 (2022).
Mikhailiuk, P. K. et al. Conformationally rigid trifluoromethyl-substituted α-amino acid designed for peptide structure analysis by solid-state 19F NMR spectroscopy. Angew. Chem. Int. Ed. 45, 5659–5661 (2006).
Mykhailiuk, P. K., Voievoda, N. M., Afonin, S., Ulrich, A. S. & Komarov, I. V. An optimized protocol for the multigram synthesis of 3-(trifluoromethyl)bicyclo[1.1.1]pent-1-ylglycine (CF3-Bpg). J. Fluorine Chem. 131, 217–220 (2010).
Kokhan, O. et al. Design, synthesis, and application of an optimized monofluorinated aliphatic label for peptide studies by solid-state 19F NMR spectroscopy. Angew. Chem. Int. Ed. 55, 14788–14792 (2016).
Bychek, R. & Mykhailiuk, P. K. A practical and scalable approach to fluoro-substituted bicyclo[1.1.1]pentanes. Angew. Chem. Int. Ed. 61, e202205103 (2022).
Pickford, H. D. et al. Rapid and scalable halosulfonylation of strain-release reagents. Angew. Chem. Int. Ed. 62, e202213508 (2023).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Iida, T. et al. Practical and facile access to bicyclo[3.1.1]heptanes: potent bioisosteres of meta-substituted benzenes. J. Am. Chem. Soc. 144, 21848–21852 (2022).
MacBean, C. (ed.) The Pesticide Manual (British Crop Production Council, 2012).
Qiao, J. X. et al. Achieving structural diversity using the perpendicular conformation of alpha-substituted phenylcyclopropanes to mimic the bioactive conformation of ortho-substituted biphenyl P4 moieties: discovery of novel, highly potent inhibitors of Factor Xa. Bioorg. Med. Chem. Lett. 18, 4118–4123 (2008).
Shinozuka, T. et al. Discovery of DS-1971a, a potent, selective NaV1.7 inhibitor. J. Med. Chem. 63, 10204–10220 (2020).
Ma, X., Han, Y. & Bennett, D. J. Selective synthesis of 1-dialkylamino-2-alkylbicyclo-[1.1.1]pentanes. Org. Lett. 22, 9133–9138 (2020).
Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl Acad. Sci. USA 118, e2108881118 (2020).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Guo, R. et al. Strain release [2π + 2σ] cycloadditions for the synthesis of bicyclo[2.1.1]hexanes initiated by energy transfer. J. Am. Chem. Soc. 144, 7988–7994 (2022).
Agasti, S. et al. A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres. Nat. Chem. 15, 535–541 (2023).
Yang, Y. et al. An intramolecular coupling approach to alkyl bioisosteres for the synthesis of multisubstituted bicycloalkyl boronates. Nat. Chem. 13, 950–955 (2021).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Liang, Y., Kleinmans, R., Daniliuc, C. G. & Glorius, F. Synthesis of polysubstituted 2-oxabicyclo[2.1.1]hexanes via visible-light-induced energy transfer. J. Am. Chem. Soc. 144, 20207–20213 (2022).
Harmata, A. S., Spiller, T. E., Sowden, M. J. & Stephenson, C. R. J. Photochemical formal (4 + 2)-cycloaddition of imine-substituted bicyclo[1.1.1]pentanes and alkenes. J. Am. Chem. Soc. 143, 21223–21228 (2021).
Chalyk, B. et al. Unexpected isomerization of oxetane-carboxylic acids. Org. Lett. 24, 4722–4728 (2022).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Ed. 59, 7161–7167 (2020).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Duboudin, J. G., Jousse, B. & Saux, A. Reactifs de grignard vinyliques γ fonctionnels: I. Reactivite des organomagnesiens vis-a-vis d’alcools α acetyleniques en presence d’halogenures cuivreux. J. Organomet. Chem. 168, 1–11 (1979).
Grygorenko, O. O., Demenko, D., Volochnyuk, D. M. & Komarov, I. V. Following Ramachandran 2: exit vector plot (EVP) analysis of disubstituted saturated rings. New J. Chem. 42, 8355–8365 (2018).
Wang, J.-R., Wang, X., Lu, L. & Mei, X. Highly crystalline forms of valsartan with superior physicochemical stability. Cryst. Growth Des. 13, 3261–3269 (2013).
Chadha, R., Bhandari, S., Haneef, J., Khullar, S. & Mandal, S. Cocrystals of telmisartan: characterization, structure elucidation, in vivo and toxicity studies. Cryst. Eng. Comm. 16, 8375–8389 (2014).
Nicolaou, K. C. et al. Synthesis and biopharmaceutical evaluation of imatinib analogues featuring unusual structural motifs. ChemMedChem 11, 31–37 (2016).
Acknowledgements
We are grateful to A. A. Tolmachov for the support; to I. Sadkova for the help with the preparation of the Supplementary Information; and to S. Shishkina for the X-ray analysis of compounds 5b and 9b. P.K.M. is also grateful to B. Heilman for proofreading the text. This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 101000893, BENOVELTY).
Author information
Authors and Affiliations
Contributions
A.D., P.G., Y.H., P.B. and P.K.M. designed the experiments. A.D., P.G., N.M.V. and G.A.-M. conducted and analysed the experiments described in this report. A.D., N.M.V., P.B. and P.K.M. prepared this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare the following competing interests: A.D., P.G. and P.K.M. are employees of a chemical supplier, Enamine. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Murugaiah Subbaiah and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Synthetic procedures, characterization of compounds (description of NMR spectra, high resolution mass spectrometry), photos of the experimental set-up of photochemical reactions, copies of NMR spectra, X-ray crystallographic data, determination of aqueous solubility, determination of lipophilicity (logD), determination of metabolic stability and determination of antifungal activity.
Supplementary Data 1
Crystallographic data for compound 5b; CCDC reference 2166325.
Supplementary Data 2
Crystallographic data for compound 9b; CCDC reference 2166326.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Denisenko, A., Garbuz, P., Voloshchuk, N.M. et al. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 15, 1155–1163 (2023). https://doi.org/10.1038/s41557-023-01222-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01222-0
This article is cited by
-
Benzocaine-N-acylindoline conjugates: synthesis and antiviral activity against Coxsackievirus B3
Medicinal Chemistry Research (2024)
-
2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
Nature Communications (2023)