Featured
-
-
Article |
 A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue
A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue
An enzyme in which a reactive unnatural amino acid functions as a catalytic residue has now been designed. Embedding an aniline side chain into the promiscuous binding pocket of a multidrug resistance regulator endowed the protein scaffold with new-to-nature activities for hydrazone and oxime formation.
- Ivana Drienovská
- , Clemens Mayer
- & Gerard Roelfes
-
Article |
 Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase
Non-aqueous homogenous biocatalytic conversion of polysaccharides in ionic liquids using chemically modified glucosidase
As a consequence of high chemical resistance and low solubility in conventional solvents, deconstructing biomass into fuels and other useful chemical building blocks remains a challenge. Now, through enzyme modification and ionic liquid solvents, it is possible to homogeneously biocatalytically convert cellulose to sugars at a rate 30 times greater than is achievable in water.
- Alex P. S. Brogan
- , Liem Bui-Le
- & Jason P. Hallett
-
Article |
 Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases
Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases
Enzymes are powerful catalysts for chemical synthesis because they are capable of providing unparalleled levels of selectivity; however, in nature they only catalyse a limited collection of reactions. Now, it has been shown that non-natural reactions that proceed via free-radical intermediates can be catalysed with high selectivity by using an exogenous photoredox catalyst in conjunction with enzymes.
- Kyle F. Biegasiewicz
- , Simon J. Cooper
- & Todd K. Hyster
-
News & Views |
 Reprogramming assembly lines
Reprogramming assembly lines
Rational engineering of biosynthetic assembly lines for production of new compounds is an attractive prospect, yet it presents many challenges. Learning from biology, some of the rules for expanding the chemical diversity of non-ribosomal peptides have been uncovered in two recent studies.
- Binuraj R. K. Menon
- & Matthew Jenner
-
Article |
 Evolving artificial metalloenzymes via random mutagenesis
Evolving artificial metalloenzymes via random mutagenesis
Proteins have the potential to serve as powerful scaffolds that control the catalytic activity and selectivity of organometallic centres; however, new methods are needed to optimize artificial metalloenzymes. Now, an efficient approach for evolving the activity and selectivity of artificial metalloenzymes has been demonstrated using dirhodium cyclopropanases. This approach does not require structural or mechanistic data to guide mutagenesis.
- Hao Yang
- , Alan M. Swartz
- & Jared C. Lewis
-
Article |
 Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells
Asymmetric transfer hydrogenation by synthetic catalysts in cancer cells
Intracellular asymmetric transfer hydrogenation catalysis using Os(II) complexes has now been demonstrated and offers a new approach for selectively killing cancer cells. Enantiomers of Os(II) arene catalysts can penetrate cell membranes enabling the reduction of pyruvate to D- or L-lactate using formate as a hydride source, with high enantioselectivity.
- James P. C. Coverdale
- , Isolda Romero-Canelón
- & Peter J. Sadler
-
Article |
 De novo design and engineering of non-ribosomal peptide synthetases
De novo design and engineering of non-ribosomal peptide synthetases
Peptides derived from non-ribosomal peptide synthetases (NRPS) are an important class of pharmaceutically relevant drugs. However, no general rules for the modification of NRPS or the generation of artificial NRPS are known. Now, a new strategy for the modification of NRPS has been developed that uses defined exchange units that are fused at specific positions connecting the condensation and adenylation domains.
- Kenan A. J. Bozhüyük
- , Florian Fleischhacker
- & Helge B. Bode
-
Article |
 A dual role for a polyketide synthase in dynemicin enediyne and anthraquinone biosynthesis
A dual role for a polyketide synthase in dynemicin enediyne and anthraquinone biosynthesis
The anthraquinone and enediyne halves of the antitumor antibiotic dynemicin A were previously thought to be assembled by two separate polyketide synthases (PKS). Now, a single polyketide synthase has been proposed to be responsible for their production, and a working model for their biosynthesis from a common octaketide intermediate has been suggested.
- Douglas R. Cohen
- & Craig A. Townsend
-
Article |
 Biocatalytic site- and enantioselective oxidative dearomatization of phenols
Biocatalytic site- and enantioselective oxidative dearomatization of phenols
Within natural product biosynthetic pathways, nature has evolved highly selective catalysts capable of complexity-generating reactions. Leveraging these tools, a suite of catalysts with complementary site- and stereoselectivity have been applied to the oxidative dearomatization of phenolic compounds, enabling one-pot transformations of phenols into various natural products.
- Summer A. Baker Dockrey
- , April L. Lukowski
- & Alison R. H. Narayan
-
News & Views |
 New functional twists for P450s
New functional twists for P450s
Two papers provide insight into the reactivity of cytochrome P450s. A direct link between electron donation and reactivity has been shown with a selenocysteine-ligated P450 compound I, whereas a serine-ligated P450 (P411) has been engineered to catalyse an intermolecular C–H amination via nitrene transfer.
- Rudi Fasan
-
Article |
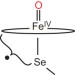 Characterization of a selenocysteine-ligated P450 compound I reveals direct link between electron donation and reactivity
Characterization of a selenocysteine-ligated P450 compound I reveals direct link between electron donation and reactivity
The oxidative prowess of cytochrome P450s has been suggested to stem from the electron-donating axial ligand. Now, a selenocysteine-ligated P450 compound I has been trapped and characterized providing an avenue to examine this hypothesis. Measurements reveal that the selenolate-ligated compound I cleaves C–H bonds more rapidly than the wild-type equivalent.
- Elizabeth L. Onderko
- , Alexey Silakov
- & Michael T. Green
-
Article |
 A reductive aminase from Aspergillus oryzae
A reductive aminase from Aspergillus oryzae
An enzyme (AspRedAm) capable of coupling carbonyls with a variety of amines in a reductive amination has now been discovered. Kinetic studies revealed that the enzyme catalysed both the imine formation step, as well as the reduction step. Structure and mutagenesis studies have highlighted essential catalytic residues and preparative scale examples have demonstrated total turnover numbers of up to 32,000.
- Godwin A. Aleku
- , Scott P. France
- & Nicholas J. Turner
-
Article |
 Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme
Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme
The intermolecular amination of C–H bonds is an enabling transformation for the synthesis of nitrogen-containing molecules; however, developing catalysts for this class of reactions is very challenging. Now, an iron-based enzyme for this reaction has been engineered, demonstrating that a protein can confer a difficult new function upon an otherwise unreactive base metal.
- Christopher K. Prier
- , Ruijie K. Zhang
- & Frances H. Arnold
-
Article |
 Catalytic diversity in self-propagating peptide assemblies
Catalytic diversity in self-propagating peptide assemblies
Simple peptides are shown to assemble into well-defined amyloid phases with paracrystalline surfaces that can catalyse reactions in an enantioselective manner. Modifying individual amino acids in the building blocks enables the structure of the assembled aggregates, and the reactions that they can catalyse, to be controlled predictably.
- Tolulope O. Omosun
- , Ming-Chien Hsieh
- & David G. Lynn
-
Article |
 Post-translational modification of ribosomally synthesized peptides by a radical SAM epimerase in Bacillus subtilis
Post-translational modification of ribosomally synthesized peptides by a radical SAM epimerase in Bacillus subtilis
Radical SAM enzymes are versatile enzymes catalysing chemically challenging reactions. Now, a radical SAM enzyme that post-translationally modifies ribosomally synthesized peptides to contain D-amino acids has been discovered in Bacillus subtilis, and its mechanism has been deciphered. These peptides, called epipeptides, efficiently inhibit bacterial growth.
- Alhosna Benjdia
- , Alain Guillot
- & Olivier Berteau
-
Article |
 Cooperative polymerization of α-helices induced by macromolecular architecture
Cooperative polymerization of α-helices induced by macromolecular architecture
The secondary and tertiary structure of a protein has profound implications on function and catalysis. Now, both the secondary and tertiary structures of a synthetic polymer have been utilized to catalyse the polymerization of N-carboxyanhydrides. Both the folding of the resulting polypeptides into α-helices and their macromolecular organization dramatically enhance the polymerization rate.
- Ryan Baumgartner
- , Hailin Fu
- & Jianjun Cheng
-
Article |
 Seven enzymes create extraordinary molecular complexity in an uncultivated bacterium
Seven enzymes create extraordinary molecular complexity in an uncultivated bacterium
Polytheonamides are giant peptide toxins produced by the uncultivated sponge bacterium Entotheonella factor. The biosynthesis of polytheonamides involves up to 50 post-translational modifications. Now, heterologous expression in Escherichia coli and Rhizobium hosts have shown that a minimalistic, iterative enzyme set introduces this exceptional molecular complexity via epimerizations, C-/N-methylations, hydroxylations, dehydration and proteolytic maturation.
- Michael F. Freeman
- , Maximilian J. Helf
- & Jörn Piel
-
Article |
 Engineering genetic circuit interactions within and between synthetic minimal cells
Engineering genetic circuit interactions within and between synthetic minimal cells
Genetic circuits are important for synthetic biology, biochemistry and bioengineering. Now, the encapsulation of genetic circuits into liposomes has been shown to enable a more modular design, the selective isolation of reactions from the environment and from each other, and the hierarchical assembly of reaction products.
- Katarzyna P. Adamala
- , Daniel A. Martin-Alarcon
- & Edward S. Boyden
-
Article |
 Self-assembly of nanoparticles into biomimetic capsid-like nanoshells
Self-assembly of nanoparticles into biomimetic capsid-like nanoshells
Biomolecular nanoscale compartments are ubiquitous in living systems. Although their formation is fairly straightforward, the same cannot be said of their inorganic counterparts. In this study, uniform nanoshells are observed self-assembling from stabilizer-free inorganic nanoparticles in water, under ambient conditions, and without the need for spherical tiling. This enables further study of inorganic prebiotic systems and compartmentalized biomimetic catalysis.
- Ming Yang
- , Henry Chan
- & Nicholas A. Kotov
-
Article |
 Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases
Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases
Despite decades of research into heme-copper oxidases, the advantages provided by copper over iron as the non-heme metal has remained unclear. Now, the preference of copper over iron has finally been explained. Copper favours faster electron transfer and higher O–O bond activation, which results in much higher oxidase activity than would be achieved by an iron equivalent.
- Ambika Bhagi-Damodaran
- , Matthew A. Michael
- & Yi Lu
-
Article |
 Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase
Emergence of a catalytic tetrad during evolution of a highly active artificial aldolase
An artificial aldolase has been optimized using an ultrahigh-throughput microfluidic screening assay. The evolved enzyme exhibits excellent stereoselectivity and broad substrate scope. Structural studies suggest that a Lys-Tyr-Asn-Tyr catalytic tetrad, which emerged during directed evolution, is responsible for the >109 rate enhancement achieved by this catalyst.
- Richard Obexer
- , Alexei Godina
- & Donald Hilvert
-
News & Views |
 Functional Frankensteins
Functional Frankensteins
An artificial esterase with no known natural structural analogues has been formed via the homo-heptameric self-assembly of a designed peptide. This esterase represents the first report of a functional catalytic triad rationally engineered into a de novo protein framework.
- Olga V. Makhlynets
- & Ivan V. Korendovych
-
Article |
 Identification of (S)-selective transaminases for the asymmetric synthesis of bulky chiral amines
Identification of (S)-selective transaminases for the asymmetric synthesis of bulky chiral amines
A motif was identified in the scaffold of an (S)-selective transaminase that enables the asymmetric synthesis of bulky chiral amines. This motif is transferable to other enzymes with as low as 70% sequence identity. The biocatalysts developed show high stereoselectivity and their synthetic potential was confirmed in preparative scale synthesis.
- Ioannis V. Pavlidis
- , Martin S. Weiß
- & Uwe T. Bornscheuer
-
Article |
 Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase
Nickel-centred proton reduction catalysis in a model of [NiFe] hydrogenase
[NiFe] hydrogenases are enzymes containing nickel and iron centres that catalyse hydrogen evolution with performances that rival those of platinum catalysts. Now, a NiFe model complex has been reported that mimics the structure and the Ni-centred hydrogen evolution activity found at the active site of [NiFe] hydrogenases.
- Deborah Brazzolotto
- , Marcello Gennari
- & Carole Duboc
-
Article |
 Installing hydrolytic activity into a completely de novo protein framework
Installing hydrolytic activity into a completely de novo protein framework
Functional catalytic triads have been designed into a hyperstable heptameric α-helical barrel protein. Twenty-one mutations were introduced to form seven Cys-His-Glu catalytic triads. The resulting protein hydrolyses p-nitrophenyl acetate with activities matching the most-efficient redesigned hydrolases based on natural protein scaffolds. This is the first example of a functional catalytic triad being engineered into a fully de novo protein.
- Antony J. Burton
- , Andrew R. Thomson
- & Derek N. Woolfson
-
Article |
 Evolution of thermophilic DNA polymerases for the recognition and amplification of C2ʹ-modified DNA
Evolution of thermophilic DNA polymerases for the recognition and amplification of C2ʹ-modified DNA
Naturally occurring DNA polymerases can amplify DNA efficiently via PCR, but they cannot utilize C2′-modified substrates to make non-natural nucleic acids. Such C2′-modified nucleic acids are of interest as they are resistant to nucleases. Now, a Stoffel fragment DNA polymerase has been evolved to transcribe C2′-modified DNA from a DNA template, reverse transcribe C2′-modified DNA back into DNA, and PCR-amplify C2′-modified DNA.
- Tingjian Chen
- , Narupat Hongdilokkul
- & Floyd E. Romesberg
-
Article |
 Carbon–sulfur bond-forming reaction catalysed by the radical SAM enzyme HydE
Carbon–sulfur bond-forming reaction catalysed by the radical SAM enzyme HydE
L-Cysteine-derived thiazolidines have now been shown to be artificial substrates of the radical SAM enzyme HydE, which converts them into S-adenosyl-L-cysteine. Carbon–sulfur bonds are formed in a concerted mechanism that involves the formation of a C-centred radical that concomitantly attacks the S atom of a thioether. This is the first example of a radical SAM enzyme that reacts directly on a sulfur atom instead of abstracting an H-atom.
- Roman Rohac
- , Patricia Amara
- & Yvain Nicolet
-
Review Article |
 Substrate channelling as an approach to cascade reactions
Substrate channelling as an approach to cascade reactions
In enzyme-catalysed metabolic pathways, substrate channelling often directs the movement of intermediates from one active site to the next. Intramolecular tunnels, electrostatic interactions and chemical swing arms pass intermediates from one enzyme to the next, enhancing pathway catalysis. Introducing mechanisms of bounded diffusion in chemical cascades can increase selectivity, transient rates and overall yield.
- Ian Wheeldon
- , Shelley D. Minteer
- & Matthew Sigman
-
Article |
 Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models
Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models
A collaborative approach between experiment and simulation has revealed a single mutation in the F/G loop of the newly described nitrating cytochrome P450 TxtE that controls loop dynamics and, more surprisingly, the regioselectivity of the reaction. This mutation is present in a subset of homologous nitrating P450s that produce a previously unidentified biosynthetic intermediate, 5-nitro-L-tryptophan.
- Sheel C. Dodani
- , Gert Kiss
- & Frances H. Arnold
-
Article |
 Self-assembling biomolecular catalysts for hydrogen production
Self-assembling biomolecular catalysts for hydrogen production
The encapsulation and stabilization of an oxygen tolerant [NiFe]-hydrogenase, sequestered within the bacteriophage P22 capsid, has now been achieved through a directed self-assembly process. Probing the catalytic activity and infrared spectroscopic signatures of the bio-inspired assembly shows that the capsid provides stability and protection to the hydrogenase cargo.
- Paul C. Jordan
- , Dustin P. Patterson
- & Trevor Douglas
-
News & Views |
 Chiral cascades
Chiral cascades
Racemic or enantiomerically pure alcohols can be converted with high yield into enantiopure chiral amines in a one-pot redox-neutral cascade process by the clever combination of an alcohol dehydrogenase and an appropriate amine dehydrogenase.
- Jian-bo Wang
- & Manfred T. Reetz
-
Article |
 Reconstitution of [Fe]-hydrogenase using model complexes
Reconstitution of [Fe]-hydrogenase using model complexes
[Fe]-hydrogenase has an iron-guanylylpyridinol cofactor and catalyses the reversible hydrogenation of a methenyl-tetrahydromethanopterin. Now, [Fe]-hydrogenase has been reconstituted using synthetic cofactor mimics. The enzyme containing a mimic with a 2-hydroxy-pyridine group was active, whereas one containing a 2-methoxy-pyridine group was inactive. This result, together with DFT computations, supports a catalytic mechanism involving the deprotonated pyridinol hydroxy group as a proton acceptor.
- Seigo Shima
- , Dafa Chen
- & Xile Hu
-
News & Views |
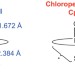 The long and the short of it
The long and the short of it
Preparation and structural characterization of the catalytic intermediates of two similar thiolate-ligated haem proteins (cytochrome P450 Compound-I and chloroperoxidase Compound-I) has explained the structural basis for the difference in their reactivity.
- Ilia G. Denisov
- & Stephen G. Sligar
-
Article |
 Asymmetric assembly of aldose carbohydrates from formaldehyde and glycolaldehyde by tandem biocatalytic aldol reactions
Asymmetric assembly of aldose carbohydrates from formaldehyde and glycolaldehyde by tandem biocatalytic aldol reactions
Forged by evolution, the natural enzymatic pathways to aldose carbohydrates are complex. Now, a biocatalytic stereoselective one-pot assembly of these carbohydrates from formaldehyde and glycolaldehyde using engineered D-fructose-6-phosphate aldolase (FSA) variants has been developed that circumvents this complexity.
- Anna Szekrenyi
- , Xavier Garrabou
- & Pere Clapés
-
Article |
 Significantly shorter Fe–S bond in cytochrome P450-I is consistent with greater reactivity relative to chloroperoxidase
Significantly shorter Fe–S bond in cytochrome P450-I is consistent with greater reactivity relative to chloroperoxidase
Cytochrome P450 (P450) and chloroperoxidase (CPO) are both thiolate-ligated haem proteins that form a ferryl radical species called compound I. P450-I is, however, significantly more reactive than CPO-I. Variable-temperature Mössbauer and X-ray absorption measurements have now shown that increased electron donation from the axial thiolate ligand in P450-I may explain its greater propensity for C–H bond activation.
- Courtney M. Krest
- , Alexey Silakov
- & Michael T. Green
-
Article |
 Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations
Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations
The reactivity of a monooxygenase (P450 PikC) has been modified through protein and substrate engineering, and applied to the oxidation of unactivated methylene C–H bonds. The protein engineering was guided by using molecular dynamics and quantum mechanical calculations to develop a predictive model for substrate scope, site selectivity and stereoselectivity of the C–H hydroxylation.
- Alison R. H. Narayan
- , Gonzalo Jiménez-Osés
- & David H. Sherman
-
-
Article |
 Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts
Supramolecular regulation of bioorthogonal catalysis in cells using nanoparticle-embedded transition metal catalysts
Regulation of bioorthogonal catalysis in living systems is challenging because of the complex intracellular environment. Now, the activity of protein-sized bioorthogonal nanozymes has been regulated by binding a supramolecular cucurbit[7]uril ‘gate-keeper’ onto the monolayer surface. This arrangement enables the controlled activation of profluorophores and prodrugs inside living cells for imaging and therapeutic applications.
- Gulen Yesilbag Tonga
- , Youngdo Jeong
- & Vincent M. Rotello
-
-
-
-
-
News & Views |
 Evolution made easy
Evolution made easy
Directed evolution is a powerful tool for the development of improved enzyme catalysts. Now, a method that enables an enzyme, its encoding DNA and a fluorescent reaction product to be encapsulated in a gel bead enables the application of directed evolution in an ultra-high-throughput format.
- Eugene J. H. Wee
- & Matt Trau
-
Article |
 Evolution of enzyme catalysts caged in biomimetic gel-shell beads
Evolution of enzyme catalysts caged in biomimetic gel-shell beads
Directed evolution has emerged as a powerful tool for the identification of improved enzyme catalysts. Now, gel-shell beads are introduced as compartments that cage an enzyme with its encoding DNA, constituting a new genotype–phenotype linkage. Screening of 107 gel-shell beads by flow cytometry leads to an improved phosphotriesterase bioremediation catalyst.
- Martin Fischlechner
- , Yolanda Schaerli
- & Florian Hollfelder
-
Article |
 Self-powered enzyme micropumps
Self-powered enzyme micropumps
Self-powered micropumps that are turned on by the presence of their respective substrates are formed from surface-immobilized, ATP-independent enzymes. Coupling substrate-sensing with transport enables the design of devices that deliver cargo in response to specific stimuli. Demonstrated here is the release of insulin at a rate proportional to ambient glucose concentration.
- Samudra Sengupta
- , Debabrata Patra
- & Ayusman Sen
-
News & Views |
 Catalytic amyloid fibrils
Catalytic amyloid fibrils
Amyloid fibrils are formed from polypeptide chains assembled into an organized fibrillar structure. Now, it has been shown that such fibrillar structures can also bind metal ions and catalyse chemical reactions.
- Tobias Aumüller
- & Marcus Fändrich
-
Article |
 Short peptides self-assemble to produce catalytic amyloids
Short peptides self-assemble to produce catalytic amyloids
Amyloid fibril formation is often catalysed by mature fibrils or other aggregates on the fibrillization pathway; however, fibrils cannot normally catalyse other chemical reactions. Here, small seven-residue peptides designed from first principles are shown to form amyloid fibrils that can efficiently catalyse ester hydrolysis.
- Caroline M. Rufo
- , Yurii S. Moroz
- & Ivan V. Korendovych
-
-

 Enzyme-powered motility in buoyant organoclay/DNA protocells
Enzyme-powered motility in buoyant organoclay/DNA protocells Harnessing haemoproteins
Harnessing haemoproteins Unnatural biocatalysts
Unnatural biocatalysts Encoding copper catalysts
Encoding copper catalysts Catalysis in compartments
Catalysis in compartments