Abstract
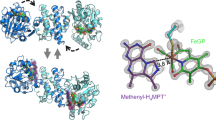
[Fe]-Hydrogenase catalyses the reversible hydrogenation of a methenyltetrahydromethanopterin substrate, which is an intermediate step during the methanogenesis from CO2 and H2. The active site contains an iron-guanylylpyridinol cofactor, in which Fe2+ is coordinated by two CO ligands, as well as an acyl carbon atom and a pyridinyl nitrogen atom from a 3,4,5,6-substituted 2-pyridinol ligand. However, the mechanism of H2 activation by [Fe]-hydrogenase is unclear. Here we report the reconstitution of [Fe]-hydrogenase from an apoenzyme using two FeGP cofactor mimics to create semisynthetic enzymes. The small-molecule mimics reproduce the ligand environment of the active site, but are inactive towards H2 binding and activation on their own. We show that reconstituting the enzyme using a mimic that contains a 2-hydroxypyridine group restores activity, whereas an analogous enzyme with a 2-methoxypyridine complex was essentially inactive. These findings, together with density functional theory computations, support a mechanism in which the 2-hydroxy group is deprotonated before it serves as an internal base for heterolytic H2 cleavage.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Vignais, P. M. & Billoud, B. Occurrence, classification, and biological function of hydrogenases: an overview. Chem. Rev. 107, 4206–4272 (2007).
Fontecilla-Camps, J. C., Volbeda, A., Cavazza, C. & Nicolet, Y. Structure/function relationships of [NiFe]- and [FeFe]-hydrogenases. Chem. Rev. 107, 4273–4303 (2007).
Lubitz, W., Ogata, H., Rudiger, O. & Reijerse, E. Hydrogenases. Chem. Rev. 114, 4081–4148 (2014).
Shima, S. et al. The crystal structure of [Fe]-hydrogenase reveals the geometry of the active site. Science 321, 572–575 (2008).
Shima, S. & Ermler, U. Structure and function of [Fe]-hydrogenase and its iron-guanylylpyridinol (FeGP) cofactor. Eur. J. Inorg. Chem. 963–972 (2011).
Buurman, G., Shima, S. & Thauer, R. K. The metal-free hydrogenase from methanogenic archaea: evidence for a bound cofactor. FEBS Lett. 485, 200–204 (2000).
Shima, S. et al. The cofactor of the iron–sulfur cluster free hydrogenase Hmd: structure of the light-inactivation product. Angew. Chem. Int. Ed. 43, 2547–2551 (2004).
Hiromoto, T. et al. The crystal structure of C176A mutated [Fe]-hydrogenase suggests an acyl–iron ligation in the active site iron complex. FEBS Lett. 583, 585–590 (2009).
Vogt, S., Lyon, E. J., Shima, S. & Thauer, R. K. The exchange activities of [Fe] hydrogenase (iron–sulfur-cluster-free hydrogenase) from methanogenic archaea in comparison with the exchange activities of [FeFe] and [NiFe] hydrogenases. J. Biol. Inorg. Chem. 13, 97–106 (2008).
Hiromoto, T., Warkentin, E., Moll, J., Ermler, U. & Shima, S. The crystal structure of an [Fe]-hydrogenase-substrate complex reveals the framework for H2 activation. Angew. Chem. Int. Ed. 48, 6457–6460 (2009).
Chen, D., Scopelliti, R. & Hu, X. [Fe]-hydrogenase models featuring acylmethylpyridinyl ligands. Angew. Chem. Int. Ed. 49, 7512–7515 (2010).
Chen, D., Scopelliti, R. & Hu, X. A five-coordinate iron center in the active site of [Fe]-hydrogenase: hints from a model study. Angew. Chem. Int. Ed. 50, 5671–5673 (2011).
Hu, B., Chen, D. & Hu, X. Synthesis and reactivity of mononuclear iron models of [Fe]-hydrogenase that contain an acylmethylpyridinol ligand. Chem. Eur. J. 20, 1677–1682 (2014).
Turrell, P. J., Wright, J. A., Peck, J. N. T., Oganesyan, V. S. & Pickett, C. J. The third hydrogenase: a ferracyclic carbamoyl with close structural analogy to the active site of Hmd. Angew. Chem. Int. Ed. 49, 7508–7511 (2010).
Schultz, K. M., Chen, D. & Hu, X. [Fe]-hydrogenase and models that contain iron–acyl ligation. Chem. Asian J. 8, 1068–1075 (2013).
Song, L. C. et al. Synthesis, structural characterization, and some properties of 2-acylmethyl-6-ester group-difunctionalized pyridine-containing iron complexes related to the active site of [Fe]-hydrogenase. Dalton Trans. 43, 8062–8071 (2014).
Royer, A. M., Salomone-Stagni, M., Rauchfuss, T. B. & Meyer-Klaucke, W. Iron acyl thiolato carbonyls: structural models for the active site of the [Fe]-hydrogenase (Hmd). J. Am. Chem. Soc. 132, 16997–17003 (2010).
Tard, C. & Pickett, C. J. Structural and functional analogues of the active sites of the [Fe]-, [NiFe]-, and [FeFe]-hydrogenases. Chem. Rev. 109, 2245–2274 (2009).
Camara, J. M. & Rauchfuss, T. B. Combining acid–base, redox and substrate binding functionalities to give a complete model for the FeFe-hydrogenase. Nature Chem. 4, 26–30 (2012).
Ogo, S. et al. A functional [NiFe] hydrogenase mimic that catalyzes electron and hydride transfer from H2 . Science 339, 682–684 (2013).
Xu, T., Chen, D. & Hu, X. Hydrogen-activating models of hydrogenases. Coord. Chem. Rev. 303, 32–41 (2015).
Shima, S., Schick, M., Ataka, K., Steinbach, K. & Linne, U. Evidence for acyl–iron ligation in the active site of [Fe]-hydrogenase provided by mass spectrometry and infrared spectroscopy. Dalton Trans. 41, 767–771 (2012).
Yang, X. Z. & Hall, M. B. Monoiron hydrogenase catalysis: hydrogen activation with the formation of a dihydrogen, Fe–Hδ–···Hδ+–O, bond and methenyl-H4MPT+ triggered hydride transfer. J. Am. Chem. Soc. 131, 10901–10908 (2009).
Finkelmann, A. R., Stiebritz, M. T. & Reiher, M. Kinetic modeling of hydrogen conversion at [Fe]-hydrogenase active-site models. J. Phys. Chem. B 117, 4806–4817 (2013).
Dey, A. Density functional theory calculations on the mononuclear non-heme iron active site of Hmd hydrogenase: role of the internal ligands in tuning external ligand binding and driving H2 heterolysis. J. Am. Chem. Soc. 132, 13892–13901 (2010).
Wodrich, M. D. & Hu, X. Electronic elements governing the binding of small molecules to a Fe-hydrogenase mimic. Eur. J. Inorg. Chem. 2013, 3993–3999 (2013).
Murray, K. A., Wodrich, M. D., Hu, X. L. & Corminboeuf, C. Toward functional type III [Fe]-hydrogenase biomimics for H2 activation: insights from computation. Chem.-Eur. J. 21, 3987–3996 (2015).
Ma, K., Zirngibl, C., Linder, D., Stetter, K. O. & Thauer, R. K. N5,N10-methylenetetrahydromethanopterin dehydrogenase (H2-forming) from the extreme thermophile Methanopyrus kandleri. Arch. Microbiol. 156, 43–48 (1991).
Zirngibl, C. et al. H2-forming methylenetetrahydromethanopterin dehydrogenase, a novel type of hydrogenase without iron–sulfur clusters in methanogenic archaea. Eur. J. Biochem. 208, 511–520 (1992).
Berggren, G. et al. Biomimetic assembly and activation of [FeFe]-hydrogenases. Nature 499, 66–69 (2013).
Esselborn, J. et al. Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic. Nature Chem. Biol. 9, 607–609 (2013).
Siebel, J. F. et al. Hybrid [FeFe]-hydrogenases with modified active sites show remarkable residual enzymatic activity. Biochemistry 54, 1474–1483 (2015).
Shima, S., Schick, M. & Tamura, H. Preparation of [Fe]-hydrogenase from methanogenic archaea. Methods Enzymol. 494, 119–137 (2011).
Bart, S. C., Lobkovsky, E. & Chirik, P. J. Preparation and molecular and electronic structures of iron(0) dinitrogen and silane complexes and their application to catalytic hydrogenation and hydrosilation. J. Am. Chem. Soc. 126, 13794–13807 (2004).
Lagaditis, P. O. et al. Iron(II) complexes containing unsymmetrical P–N–P′ pincer ligands for the catalytic asymmetric hydrogenation of ketones and imines. J. Am. Chem. Soc. 136, 1367–1380 (2014).
Shima, S. & Ataka, K. Isocyanides inhibit [Fe]-hydrogenase with very high affinity. FEBS Lett. 585, 353–356 (2011).
Lyon, E. J. et al. Carbon monoxide as an intrinsic ligand to iron in the active site of the iron–sulfur-cluster-free hydrogenase H2-forming methylenetetrahydromethanopterin dehydrogenase as revealed by infrared spectroscopy. J. Am. Chem. Soc. 126, 14239–14248 (2004).
Korbas, M. et al. The iron–sulfur cluster-free hydrogenase (Hmd) is a metalloenzyme with a novel iron binding motif. J. Biol. Chem. 281, 30804–30813 (2006).
Tamura, H. et al. Crystal structures of [Fe]-hydrogenase in complex with inhibitory isocyanides: implications for H2-activation site. Angew. Chem. Int. Ed. 52, 9656–9659 (2013).
Finkelmann, A. R., Senn, H. M. & Reiher, M. Hydrogen-activation mechanism of [Fe] hydrogenase revealed by multi-scale modeling. Chem. Sci. 5, 4474–4482 (2014).
Zhao, Y. & Truhlar, D. G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Zhao, Y. & Truhlar, D. G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 41, 157–167 (2008).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Gaussian 09, revision D.01 (Gaussian, Inc., Wallingford, Connecticut, 2009).
Steinmann, S. N. & Corminboeuf, C. Comprehensive bench marking of a density-dependent dispersion correction. J. Chem. Theory Comp. 7, 3567–3577 (2011).
Becke, A. D. Density-functional thermochemistry. 3. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Lee, C. T., Yang, W. T. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron-density. Phys. Rev. B 37, 785–789 (1988).
ADF2013 (Scientific Computing and Modelling, Amsterdam, 2013).
Klamt, A. The COSMO and COSMO-RS solvation models. WIREs Comput. Mol. Sci. 1, 699–709 (2011).
Acknowledgements
We thank R. Thauer for discussions and helpful suggestions. C. Corminboeuf and the Laboratory for Computational Molecular Design at the EPFL are acknowledged for providing computational resources. This work was supported by grants from the Max Planck Society (to R. Thauer) and for the PRESTO program from the Japan Science and Technology Agency to S. Shima, a grant from the National Natural Science Foundation of China (No. 21302028) to D. Chen and grants from the Swiss National Science Foundation (200020_134473/1 and 200020_152850/1) to X. Hu.
Author information
Authors and Affiliations
Contributions
S.S. and X.H. directed the research. S.S., D.C. and X.H. designed the study. D.C., T.X. and K.M.S. synthesized the model compounds. S.S. reconstituted and characterized the semisynthetic [Fe]-hydrogenase. J.K. performed the matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis. K.A. performed the infrared spectroscopy with S.S. and T.F. M.D.W. carried out the computations. S.S. and X.H. wrote the manuscript with contributions from all the co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1236 kb)
Rights and permissions
About this article
Cite this article
Shima, S., Chen, D., Xu, T. et al. Reconstitution of [Fe]-hydrogenase using model complexes. Nature Chem 7, 995–1002 (2015). https://doi.org/10.1038/nchem.2382
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2382
This article is cited by
-
Stepwise assembly of the active site of [NiFe]-hydrogenase
Nature Chemical Biology (2023)
-
Biomimetic asymmetric catalysis
Science China Chemistry (2023)
-
Methanogenesis involves direct hydride transfer from H2 to an organic substrate
Nature Reviews Chemistry (2020)
-
The atomic-resolution crystal structure of activated [Fe]-hydrogenase
Nature Catalysis (2019)
-
A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor
Nature Chemistry (2019)