Abstract
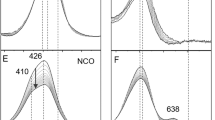
Cytochrome P450 (P450) and chloroperoxidase (CPO) are thiolate-ligated haem proteins that catalyse the activation of carbon hydrogen bonds. The principal intermediate in these reactions is a ferryl radical species called compound I. P450 compound I (P450-I) is significantly more reactive than CPO-I, which only cleaves activated C–H bonds. To provide insight into the differing reactivities of these intermediates, we examined CPO-I and P450-I using variable-temperature Mössbauer and X-ray absorption spectroscopies. These measurements indicate that the Fe–S bond is significantly shorter in P450-I than in CPO-I. This difference in Fe–S bond lengths can be understood in terms of variations in the hydrogen-bonding patterns within the ‘cys-pocket’ (a portion of the proximal helix that encircles the thiolate ligand). Weaker hydrogen bonding in P450-I results in a shorter Fe–S bond, which enables greater electron donation from the axial thiolate ligand. This observation may in part explain P450's greater propensity for C–H bond activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Groves, J. T. Enzymatic C–H bond activation using push to get pull. Nature Chem. 6, 89–91 (2014).
Dawson, J. H. Probing structure–function relations in heme-containing oxygenases and peroxidases. Science 240, 433–439 (1988).
Green, M. T., Dawson, J. H. & Gray, H. B. Oxoiron(IV) in chloroperoxidase compound II is basic: implications for P450 chemistry. Science 304, 1653–1656 (2004).
Green, M. T. C–H bond activation in heme proteins: the role of thiolate ligation in cytochrome P450. Curr. Opin. Chem. Biol. 13, 84–88 (2009).
Rittle, J. & Green, M. T. Cytochrome P450 compound I: capture, characterization, and C–H bond activation kinetics. Science 330, 933–937 (2010).
Yosca, T. H. et al. Iron(IV)hydroxide pKa and the role of thiolate ligation in C-H bond activation by cytochrome P450. Science 342, 825–829 (2013).
Krest, C. M. et al. Reactive intermediates in cytochrome P450 catalysis. J. Biol. Chem. 288, 17074–17081 (2013).
McQuarters, A. B., Wolf, M. W., Hunt, A. P. & Lehnert, N. 1958–2014: after 56 years of research, cytochrome P450 reactivity is finally explained. Angew. Chem. Int. Ed. 53, 4750–4752 (2014).
Jung, C. The mystery of cytochrome P450 compound I: a mini-review dedicated to Klaus Ruckpaul. Biochim. Biophys. Acta. 1814, 46–57 (2011).
Gross, Z. & Nimri, S. A pronounced axial ligand effect on the reactivity of oxoiron(IV) porphyrin cation radicals. Inorg. Chem. 33, 1731–1732 (1994).
Song, W. J., Ryu, Y. O., Song, R. & Nam, W. Oxoiron(IV) porphyrin π-cation radical complexes with a chameleon behavior in cytochrome P450 model reactions. J. Biol. Inorg. Chem. 10, 294–304 (2005).
Kamachi, T., Kouno, T., Nam, W. & Yoshizawa, K. How axial ligands control the reactivity of high-valent iron(IV)–oxo porphyrin π-cation radicals in alkane hydroxylation: a computational study. J. Inorg. Biochem. 100, 751–754 (2006).
Kang, Y. et al. Enhanced reactivities of iron(IV)-oxo porphyrin π-cation radicals in oxygenation reactions by electron-donating axial ligands. Chem. Eur. J. 15, 10039–10046 (2009).
Prokop, K. A., de Visser, S. P. & Goldberg, D. P. Unprecedented rate enhancements of hydrogen-atom transfer to a manganese(V)–oxo corrolazine complex. Angew. Chem. Int. Ed. 49, 5091–5095 (2010).
Urano, Y., Higuchi, T., Hirobe, M. & Nagano, T. A pronounced axial thiolate ligand effect on the reactivity of the high valent oxo-iron porphyrin intermediate. FASEB J. 11, A817–A817 (1997).
Ohno, T. et al. Remarkable axial thiolate ligand effect on the oxidation of hydrocarbons by active intermediate of iron porphyrin and cytochrome P450. J. Inorg. Biochem. 82, 123–125 (2000).
van Rantwijk, F. & Sheldon, R. A. Selective oxygen transfer catalysed by heme peroxidases: synthetic and mechanistic aspects. Curr. Opin. Biotechnol. 11, 554–564 (2000).
Rutter, R. et al. Chloroperoxidase compound I: electron paramagnetic resonance and Mössbauer studies. Biochemistry 23, 6809–6816 (1984).
Stone, K. L., Behan, R. K. & Green, M. T. X-ray absorption spectroscopy of chloroperoxidase compound I: insight into the reactive intermediate of P450 chemistry. Proc. Natl Acad. Sci. USA 102, 16563–16565 (2005).
Sundaramoorthy, M., Terner, J. & Poulos, T. L. The crystal structure of chloroperoxidase: a heme peroxidase–cytochrome P450 functional hybrid. Structure 3, 1367–1377 (1995).
Poulos, T. L., Finzel, B. C. & Howard, A. J. High-resolution crystal-structure of cytochrome-P450cam. J. Mol. Biol. 195, 687–700 (1987).
Zaks, A. & Dodds, D. R. Chloroperoxidase-catalyzed asymmetric oxidations—substrate-specificity and mechanistic study. J. Am. Chem. Soc. 117, 10419–10424 (1995).
Zhang, R., Nagraj, N., Lansakara, D. S. P., Hager, L. P. & Newcomb, M. Kinetics of two-electron oxidations by the compound I derivative of chloroperoxidase, a model for cytochrome P450 oxidants. Org. Lett. 8, 2731–2734 (2006).
Ueyama, N., Nishikawa, N., Yamada, Y., Okamura, T. & Nakamura, A. Cytochrome P-450 model (porphinato)(thiolato)iron(III) complexes with single and double NH···S hydrogen bonds at the thiolate site. J. Am. Chem. Soc. 118, 12826–12827 (1996).
Ueyama, N. et al. Synthesis and properties of octaethylporphinato(arenethiolato)iron(III) complexes with intramolecular NH–S hydrogen bond: chemical function of the hydrogen bond. Inorg. Chem. 37, 2415–2421 (1998).
Suzuki, N. et al. Novel iron porphyrin–alkanethiolate complex with intramolecular NH···S hydrogen bond: synthesis, spectroscopy, and reactivity. J. Am. Chem. Soc. 121, 11571–11572 (1999).
Ueno, T. et al. Role of the invariant peptide fragment forming NH···S hydrogen bonds in the active site of cytochrome P-450 and chloroperoxidase: synthesis and properties of Cys-containing peptide Fe(III) and Ga(III) (octaethylporphinato) complexes as models. Inorg. Chem. 38, 1199–1210 (1999).
Yoshioka, S. et al. Roles of the proximal hydrogen bonding network in cytochrome P450(cam)-catalyzed oxygenation. J. Am. Chem. Soc. 124, 14571–14579 (2002).
Dey, A. et al. Sulfur K-edge XAS and DFT calculations on P450 model complexes: effects of hydrogen bonding on electronic structure and redox potentials. J. Am. Chem. Soc. 127, 12046–12053 (2005).
Matsumura, H. et al. Modulation of redox potential and alteration in reactivity via the peroxide shunt pathway by mutation of cytochrome P450 around the proximal heme ligand. Biochemistry 47, 4834–4842 (2008).
Dey, A. et al. S K-edge XAS and DFT calculations on cytochrome P450: covalent and ionic contributions to the cysteine-Fe bond and their contribution to reactivity. J. Am. Chem. Soc. 131, 7869–7878 (2009).
Galinato, M. G. I., Spolitak, T., Ballou, D. P. & Lehnert, N. Elucidating the role of the proximal cysteine hydrogen-bonding network in ferric cytochrome P450cam and corresponding mutants using magnetic circular dichroism spectroscopy. Biochemistry 50, 1053–1069 (2011).
Lehnert, N. Elucidating second coordination sphere effects in heme proteins using low-temperature magnetic circular dichroism spectroscopy. J. Inorg. Biochem. 110, 83–93 (2012).
Park, S. Y. et al. Thermophilic cytochrome P450 (CYP119) from Sulfolobus solfataricus: high resolution structure and functional properties. J. Inorg. Biochem. 91, 491–501 (2002).
Beitlich, T., Kuhnel, K., Schulze-Briese, C., Shoeman, R. L. & Schlichting, I. Cryoradiolytic reduction of crystalline heme proteins: analysis by UV–vis spectroscopy and X-ray crystallography. J. Synchrotron Radiat. 14, 11–23 (2007).
Green, M. T. Imidazole-ligated compound I intermediates: the effects of hydrogen bonding. J. Am. Chem. Soc. 122, 9495–9499 (2000).
Green, M. T. The structure and spin coupling of catalase compound I: a study of noncovalent effects. J. Am. Chem. Soc. 123, 9218–9219 (2001).
Weiss, R. et al. Delocalization over the heme and the axial ligands of one of the two oxidizing equivalents stored above the ferric state in the peroxidase and catalase compound-I intermediates: indirect participation of the proximal axial ligand of iron in the oxidation reactions catalyzed by heme-based peroxidases and catalases? J. Biol. Inorg. Chem. 1, 377–383 (1996).
Green, M. T. Evidence for sulfur-based radicals in thiolate compound I intermediates. J. Am. Chem. Soc. 121, 7939–7940 (1999).
Schulz, C. E., Nyman, P. & Debrunner, P. G. Spin fluctuations of paramagnetic iron centers in proteins and model complexes: Mössbauer and EPR results. J. Chem. Phys. 87, 5077–5091 (1987).
Winkler, H., Schulz, C. & Debrunner, P. G. Spin fluctuation rates from Mössbauer spectra of high-spin ferrous rubredoxin. Phys. Lett. A 69, 360–363 (1979).
Chandrasekaran, P. et al. Prediction of high-valent iron K-edge absorption spectra by time-dependent density functional theory. Dalton Trans. 40, 11070–11079 (2011).
Westre, T. E. et al. A multiplet analysis of Fe K-edge 1s→ 3d pre-edge features of iron complexes. J. Am. Chem. Soc. 119, 6297–6314 (1997).
Spiro, T. G. & Wasbotten, I. H. CO as a vibrational probe of heme protein active sites. J. Inorg. Biochem. 99, 34–44 (2005).
Mak, P. J., Yang, Y. T., Im, S., Waskell, L. A. & Kincaid, J. R. Experimental documentation of the structural consequences of hydrogen-bonding interactions to the proximal cysteine of a cytochrome P450. Angew. Chem. Int. Ed. 51, 10403–10407 (2012).
Hollenberg, P. F., Hager, L. P., Blumberg, W. E. & Peisach, J. An electron paramagnetic resonance study of the high and low spin forms of chloroperoxidase. J. Biol. Chem. 255, 4801–4807 (1980).
Paulat, F. & Lehnert, N. Electronic structure of ferric heme nitrosyl complexes with thiolate coordination. Inorg. Chem. 46, 1547–1549 (2007).
Williams-Smith, D. L., Bray, R. C., Barber, M. J., Tsopanakis, A. D. & Vincent, S. P. Changes in apparent pH on freezing aqueous buffer solutions and their relevance to biochemical electron-paramagnetic-resonance spectroscopy. Biochem. J. 167, 593–600 (1977).
George, G. N. EXAFSPAK; http://ssrl.slac.stanford.edu/exafspak.html (2000).
Acknowledgements
This work was supported by the National Institutes of Health (NIH, R01-GM101390). The authors thank M. Latimer and E. Nelson for on-site assistance at the synchrotron. Use of the Stanford Synchrotron Radiation Lightsource (SSRL), SLAC National Accelerator Laboratory, is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences (contract no. DE-AC02-76SF00515). The SSRL Structural Molecular Biology Program is supported by the DOE Office of Biological and Environmental Research and by the NIH, National Institute of General Medical Sciences (including P41GM103393).
Author information
Authors and Affiliations
Contributions
M.T.G. designed the experiments. M.T.G. wrote the manuscript with input from the other authors. C.M.K., T.H.Y., E.L.O. and J.C.C. were responsible for EXAFS data collection and analyses. C.M.K. and A.S. were responsible for the VTM measurements. A.S. performed the VTM analyses. J.R. collected and analysed the kinetic data. C.M.K., T.H.Y. and E.L.O. were responsible for sample preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 1013 kb)
Rights and permissions
About this article
Cite this article
Krest, C., Silakov, A., Rittle, J. et al. Significantly shorter Fe–S bond in cytochrome P450-I is consistent with greater reactivity relative to chloroperoxidase. Nature Chem 7, 696–702 (2015). https://doi.org/10.1038/nchem.2306
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2306
This article is cited by
-
Chemodivergent C(sp3)–H and C(sp2)–H cyanomethylation using engineered carbene transferases
Nature Catalysis (2023)
-
Catalytic Mechanisms and Active Species of Benzene Hydroxylation Reaction System Based on Fe-Based Enzyme-Mimetic Structure
Catalysis Letters (2023)
-
A safety cap protects hydrogenase from oxygen attack
Nature Communications (2021)
-
EPR of Compound I: An Illustrated Revision of the Theoretical Model
Applied Magnetic Resonance (2020)
-
Characterization of a selenocysteine-ligated P450 compound I reveals direct link between electron donation and reactivity
Nature Chemistry (2017)