Abstract
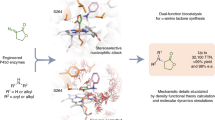
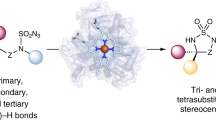
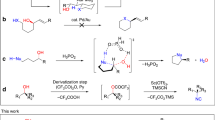
C–H bonds are ubiquitous structural units of organic molecules. Although these bonds are generally considered to be chemically inert, the recent emergence of methods for C–H functionalization promises to transform the way synthetic chemistry is performed. The intermolecular amination of C–H bonds represents a particularly desirable and challenging transformation for which no efficient, highly selective, and renewable catalysts exist. Here we report the directed evolution of an iron-containing enzymatic catalyst—based on a cytochrome P450 monooxygenase—for the highly enantioselective intermolecular amination of benzylic C–H bonds. The biocatalyst is capable of up to 1,300 turnovers, exhibits excellent enantioselectivities, and provides access to valuable benzylic amines. Iron complexes are generally poor catalysts for C–H amination: in this catalyst, the enzyme's protein framework confers activity on an otherwise unreactive iron-haem cofactor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Hartwig, J. F. Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 138, 2–24 (2016).
Godula, K. & Sames, D. C–H bond functionalization in complex organic synthesis. Science 312, 67–72 (2006).
Yamaguchi, J., Yamaguchi, A. D. & Itami, K. C–H bond functionalization: emerging synthetic tools for natural products and pharmaceuticals. Angew. Chem. Int. Ed. 51, 8960–9009 (2012).
Bertini, I., Gray, H. B., Lippard, S. J. & Valentine, J. S. (eds) Bioinorganic Chemistry (University Science Books, 1994).
Zalatan, D. N. & Du Bois, J. Metal-catalyzed oxidations of C–H to C–N bonds. Top. Curr. Chem. 292, 347–378 (2010).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Collet, F., Lescot, C. & Dauban, P. Catalytic C–H amination: the stereoselectivity issue. Chem. Soc. Rev. 40, 1926–1936 (2011).
Wu, W.-T., Yang, Z.-P. & You, S.-L. in Asymmetric Functionalization of C–H Bonds (ed. You, S.-L.) 1–66 (RSC Catalysis Series No. 25, 2015).
Jeffrey, J. L. & Sarpong, R. Intramolecular C(sp3)–H amination. Chem. Sci. 4, 4092–4106 (2013).
Ochiai, M., Miyamoto, K., Kaneaki, T., Hayashi, S. & Nakanishi, W. Highly regioselective amination of unactivated alkanes by hypervalent sulfonylimino-λ3-bromane. Science 332, 448–451 (2011).
Hennessy, E. T., Liu, R. Y., Iovan, D. A., Duncan, R. A. & Betley, T. A. Iron-mediated intermolecular N-group transfer chemistry with olefinic substrates. Chem. Sci. 5, 1526–1532 (2014).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Huang, X., Bergsten, T. M. & Groves, J. T. Manganese-catalyzed late-stage aliphatic C–H azidation. J. Am. Chem. Soc. 137, 5300–5303 (2015).
Michaudel, Q., Thevenet, D. & Baran, P. S. Intermolecular Ritter-type C–H amination of unactivated sp3 carbons. J. Am. Chem. Soc. 134, 2547–2550 (2012).
Nägeli, I. et al. Rhodium(II)-catalyzed CH Insertions with {[(4-Nitrophenyl)sulfonyl]imino}phenyl-λ3-iodane. Helv. Chim. Acta 80, 1087–1105 (1997).
Yamawaki, M., Tsutsui, H., Kitagaki, S., Anada, M. & Hashimoto, S. Dirhodium(II) tetrakis[N-tetrachlorophthaloyl-(S)-tert-leucinate]: a new chiral Rh(II) catalyst for enantioselective amidation of C–H bonds. Tetrahedron Lett. 43, 9561–9564 (2002).
Reddy, R. P. & Davies, H. M. L. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org. Lett. 8, 5013–5016 (2006).
Nishioka, Y., Uchida, T. & Katsuki, T. Enantio- and regioselective intermolecular benzylic and allylic C–H bond amination. Angew. Chem. Int. Ed. 52, 1739–1742 (2013).
Zhou, X.-G., Yu, X.-Q., Huang, J.-S. & Che, C.-M. Asymmetric amidation of saturated C–H bonds catalysed by chiral ruthenium and manganese porphyrins. Chem. Commun. 2377–2378 (1999).
Kohmura, Y. & Katsuki, T. Mn(salen)-catalyzed enantioselective C–H amination. Tetrahedron Lett. 42, 3339–3342 (2001).
Liang, C. et al. Efficient diastereoselective intermolecular rhodium-catalyzed C–H amination. Angew. Chem. Int. Ed. 45, 4641–4644 (2006).
Urlacher, V. B. & Girhard, M. Cytochrome P450 monooxygenases: an update on perspectives for synthetic application. Trends Biotechnol. 30, 26–36 (2012).
Podust, L. M. & Sherman, D. H. Diversity of P450 enzymes in the biosynthesis of natural products. Nat. Prod. Rep. 29, 1251–1266 (2012).
Both, P. et al. Whole-cell biocatalysts for stereoselective C–H amination reactions. Angew. Chem. Int. Ed. 55, 1511–1513 (2016).
Schrewe, M., Ladkau, N., Bühler, B. & Schmid, A. Direct terminal alkylamino-functionalization via multistep biocatalysis in one recombinant whole-cell catalyst. Adv. Synth. Catal. 355, 1693–1697 (2013).
McIntosh, J. A. et al. Enantioselective intramolecular C–H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew. Chem. Int. Ed. 52, 9309–9312 (2013).
Hyster, T. K., Farwell, C. C., Buller, A. R., McIntosh, J. A. & Arnold, F. H. Enzyme-controlled nitrogen-atom transfer enables regiodivergent C–H amination. J. Am. Chem. Soc. 136, 15505–15508 (2014).
Farwell, C. C., Zhang, R. K., McIntosh, J. A., Hyster, T. K. & Arnold, F. H. Enantioselective enzyme-catalyzed aziridination enabled by active-site evolution of a cytochrome P450. ACS Cent. Sci. 1, 89–93 (2015).
Prier, C. K., Hyster, T. K., Farwell, C. C., Huang, A. & Arnold, F. H. Asymmetric enzymatic synthesis of allylic amines: a sigmatropic rearrangement strategy. Angew. Chem. Int. Ed. 55, 4711–4715 (2016).
Singh, R., Bordeaux, M. & Fasan, R. P450-catalyzed intramolecular sp3 C–H amination with arylsulfonyl azide substrates. ACS Catal. 4, 546–552 (2014).
Bordeaux, M., Singh, R. & Fasan, R. Intramolecular C(sp3)–H amination of arylsulfonyl azides with engineered and artificial myoglobin-based catalysts. Bioorg. Med. Chem. 22, 5697–5704 (2014).
Svastits, E. W., Dawson, J. H., Breslow, R. & Gellman, S. H. Functionalized nitrogen atom transfer catalyzed by cytochrome P-450. J. Am. Chem. Soc. 107, 6427–6428 (1985).
Coelho, P. S. et al. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol. 9, 485–487 (2013).
Whitehouse, C. J. C., Bell, S. G. & Wong, L.-L. P450BM3 (CYP102A1): connecting the dots. Chem. Soc. Rev. 41, 1218–1260 (2012).
Barniol-Xicota, M., Leiva, R., Escolano, C. & Vázquez, S. Synthesis of cinacalcet: an enantiopure active pharmaceutical ingredient (API). Synthesis 48, 783–803 (2016).
Bloom, J. D., Labthavikul, S. T., Otey, C. R. & Arnold, F. H. Protein stability promotes evolvability. Proc. Natl Acad. Sci. USA 103, 5869–5874 (2006).
Ankner, T. & Hilmersson, G. Instantaneous deprotection of tosylamides and esters with SmI2/Amine/Water. Org. Lett. 11, 503–506 (2009).
Poulos, T. L. Cytochrome P450. Curr. Opin. Struct. Biol. 5, 767–774 (1995).
Li, H. & Poulos, T. L. The structure of the cytochrome p450BM-3 haem domain complexed with the fatty acid substrate, palmitoleic acid. Nat. Struct. Biol. 4, 140–146 (1997).
Roiban, G.-D. & Reetz, M. T. Expanding the toolbox of organic chemists: directed evolution of P450 monooxygenases as catalysts in regio- and stereoselective oxidative hydroxylation. Chem. Commun. 51, 2208–2224 (2015).
Key, H. M., Dydio, P., Clark, D. S. & Hartwig, J. F. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 534, 534–537 (2016).
Srivastava, P., Yang, H., Ellis-Guardiola, K. & Lewis, J. C. Engineering a dirhodium artificial metalloenzyme for selective olefin cyclopropanation. Nat. Commun. 6, 7789 (2015).
Hyster, T. K., Knörr, L., Ward, T. R. & Rovis, T. Biotinylated Rh(III) complexes in engineered streptavidin for accelerated asymmetric C–H activation. Science 338, 500–503 (2012).
Dydio, P., Key, H. M., Hayashi, H., Clark, D. S. & Hartwig, J. F. Chemoselective, enzymatic C–H bond amination catalyzed by a cytochrome P450 containing an Ir(Me)-PIX cofactor. J. Am. Chem. Soc. 139, 1750–1753 (2017).
Acknowledgements
Our research is supported by the National Science Foundation, Division of Molecular and Cellular Biosciences (grant MCB-1513007) and by funds from the American Recovery and Reinvestment Act (ARRA) through the National Institutes of Health Shared Instrumentation Grant Program (S10RR027203). C.K.P. thanks the Resnick Sustainability Institute for a postdoctoral fellowship. R.K.Z. was supported by a National Science Foundation Graduate Research Fellowship (NSF GRFP; DGE-1144469), is a trainee in the Caltech Biotechnology Leadership Program, and has received financial support from the Donna and Benjamin M. Rosen Bioengineering Center. A.R.B. is funded by a Ruth Kirschstein NIH Postdoctoral Fellowship F32G110851. We thank S. Virgil, J. Kaiser, and R. D. Lewis for experimental assistance, and O. F. Brandenberg, S. C. Hammer, and S. B. J. Kan for helpful discussion and comments on the manuscript.
Author information
Authors and Affiliations
Contributions
C.K.P. and R.K.Z. designed, carried out, and analysed all amination experiments, with F.H.A. providing guidance. C.K.P., R.K.Z. and S.B.-C. obtained protein crystals. R.K.Z. and A.R.B. solved the crystal structure. C.K.P. and F.H.A. wrote the manuscript with input from all of the authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 5564 kb)
Rights and permissions
About this article
Cite this article
Prier, C., Zhang, R., Buller, A. et al. Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme. Nature Chem 9, 629–634 (2017). https://doi.org/10.1038/nchem.2783
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2783
This article is cited by
-
Reaction engineering blocks ether cleavage for synthesizing chiral cyclic hemiacetals catalyzed by unspecific peroxygenase
Nature Communications (2024)
-
Chemodivergent C(sp3)–H and C(sp2)–H cyanomethylation using engineered carbene transferases
Nature Catalysis (2023)
-
Stereoselective construction of β-, γ- and δ-lactam rings via enzymatic C–H amidation
Nature Catalysis (2023)
-
Biocatalytic, stereoconvergent alkylation of (Z/E)-trisubstituted silyl enol ethers
Nature Synthesis (2023)
-
Reaction pathway engineering converts a radical hydroxylase into a halogenase
Nature Chemical Biology (2022)