Featured
-
-
News & Views |
 Assembling the pieces to improve catalysis
Assembling the pieces to improve catalysis
Understanding the ways by which metal-containing catalysts carry out a reaction is a chemical puzzle. Now, investigations of a multi-metallic molecular system uncover how the self-assembly of molecular catalysts facilitates cooperation between active species and improves the conversion of water to hydrogen gas.
- Ana Sonea
- & Jeffrey J. Warren
-
Article |
 Deprotonated 2-thiolimidazole serves as a metal-free electrocatalyst for selective acetylene hydrogenation
Deprotonated 2-thiolimidazole serves as a metal-free electrocatalyst for selective acetylene hydrogenation
Although metal-free catalysts, featuring defined active sites, represent alternatives to scarce or problematic metals, metal-free compounds rarely show activities as promising as metal-based materials. Now deprotonated 2-thiolimidazole is shown to serve as a metal-free electrocatalyst for selective acetylene hydrogenation and achieves competitive performances with metal-based catalysts.
- Lei Zhang
- , Rui Bai
- & Jian Zhang
-
Article
| Open Access Upcycling of polyethylene to gasoline through a self-supplied hydrogen strategy in a layered self-pillared zeolite
Upcycling of polyethylene to gasoline through a self-supplied hydrogen strategy in a layered self-pillared zeolite
The development of new methodologies to convert plastics into fuels without relying on noble metal-based catalysts is desirable. Now it is shown that a layered self-pillared zeolite enables the conversion of polyethylene to gasoline with a selectivity of 99% and yields of >80% without the need to use external hydrogen.
- Ziyu Cen
- , Xue Han
- & Buxing Han
-
Article
| Open Access An air- and moisture-stable ruthenium precatalyst for diverse reactivity
An air- and moisture-stable ruthenium precatalyst for diverse reactivity
Despite the widespread utility of ruthenium catalysts, many protocols for their use require high temperatures or light irradiation. Now, the synthesis of an air- and moisture-stable ruthenium precatalyst has been reported. This versatile catalyst drives an array of transformations and enables rapid screening and optimization of reactions, revealing previously unknown in situ generated ruthenium complexes.
- Gillian McArthur
- , Jamie H. Docherty
- & Igor Larrosa
-
Article |
 Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer
Asymmetric photoredox catalytic formal de Mayo reaction enabled by sensitization-initiated electron transfer
The redox properties of visible-light-absorbing photosensitizers are limited by the energy of visible photons, but methods using sensitization-initiated electron transfer have recently been developed to address these challenges. Now a multiphoton dual-catalyst strategy has been used to enable the enantioselective de Mayo reaction for the synthesis of enantioenriched 1,5-diketones.
- Xin Sun
- , Yilin Liu
- & Zhiyong Jiang
-
News & Views |
 Copper catalysed asymmetric amination
Copper catalysed asymmetric amination
Chiral amines possessing a stereogenic carbon atom bearing three carbon substituents and one nitrogen substituent are challenging structural motifs to prepare enantioselectively. Now, such motifs have been accessed in high enantiopurities by asymmetric Cu-catalysed propargylic amination using sterically confined ligands.
- Joshua D. Sieber
-
Article |
 Catalyst self-assembly accelerates bimetallic light-driven electrocatalytic H2 evolution in water
Catalyst self-assembly accelerates bimetallic light-driven electrocatalytic H2 evolution in water
Although the light-driven generation of hydrogen from water is a promising approach to renewable fuels, the H–H bond formation step represents a persistent mechanistic question. Now light-harvesting molecular catalysts have been shown to self-assemble into nanoscale aggregates that feature improved efficiency for photoelectrochemical H2 evolution.
- Isaac N. Cloward
- , Tianfei Liu
- & Alexander J. M. Miller
-
Article |
 Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Enantioenriched α-disubstituted α-ethynylamines are valuable synthons to chiral α-tertiary amines and azacycles, but their facile access remains challenging. Now, sterically confined pyridinebisoxazoline ligands have been developed to facilitate highly enantioselective Cu(I)-catalysed propargylic amination of both aliphatic and aryl ketone-derived propargylic carbonates to give α-tertiary ethynylamines. Related tandem sequences are reported to synthesize quaternary azacycles.
- Zheng Zhang
- , Ying Sun
- & Jian Zhou
-
Article
| Open Access Cage escape governs photoredox reaction rates and quantum yields
Cage escape governs photoredox reaction rates and quantum yields
The spontaneous recombination of photogenerated radicals surrounded by solvent molecules is an important energy-wasting elementary step in photoredox reactions. Now the decisive role that cage escape plays in these reactions is shown in three benchmark photocatalytic reactions, with quantitative correlations observed between photoredox product formation rates and cage escape quantum yields.
- Cui Wang
- , Han Li
- & Oliver S. Wenger
-
In Your Element |
 A blueprint for catalysis
A blueprint for catalysis
Ciro Romano, Jack I. Mansell, and David J. Procter have explored the versatility and selectivity of samarium diiodide, and its use as a radical relay catalyst.
- Ciro Romano
- , Jack I. Mansell
- & David J. Procter
-
Perspective |
 The impact of UV light on synthetic photochemistry and photocatalysis
The impact of UV light on synthetic photochemistry and photocatalysis
Although generally perceived as an old-fashioned and unselective tool to build molecules, the photochemistry community is now re-discovering the power of UV light and is using key mechanistic information to develop new catalytic processes driven by visible light. This Perspective discusses the progress and impact of UV light in organic synthesis.
- Giulio Goti
- , Kavyasree Manal
- & Luca Dell’Amico
-
Article |
 Visualization of CO2 electrolysis using optical coherence tomography
Visualization of CO2 electrolysis using optical coherence tomography
Electrolysers can upgrade CO2 into high-value chemicals, but there are few tools capable of tracking the reactions that occur within these devices during operation. Now an electrolysis optical coherence tomography platform has been developed to visualize the electrochemical conversion of CO2 to CO, plus the movement of components, within the device.
- Xin Lu
- , Chris Zhou
- & Curtis P. Berlinguette
-
Research Briefing |
 Carbon isotope exchange for pharmaceutical radiolabelling through metal-catalysed functional group metathesis
Carbon isotope exchange for pharmaceutical radiolabelling through metal-catalysed functional group metathesis
A method for carbon isotope exchange involving a metal-catalysed metathesis reaction of in situ formed acyl chlorides is demonstrated. The platform provides access to 13C- or 14C-enriched carboxylic acids, including natural products and pharmaceuticals, without the need for radioactive gases, using a single carboxylic acid carbon donor.
-
Article |
 A catalytic process enables efficient and programmable access to precisely altered indole alkaloid scaffolds
A catalytic process enables efficient and programmable access to precisely altered indole alkaloid scaffolds
New drug leads can be developed through modification of a natural product’s framework, but this is possible only if the compound is abundant and contains modifiable moieties. Now a strategy is introduced for accessing a scarce indole alkaloid and several expanded, contracted and distorted analogues, one of which shows anti-cancer activity.
- Youming Huang
- , Xinghan Li
- & Amir H. Hoveyda
-
Article |
 A metal-catalysed functional group metathesis approach to the carbon isotope labelling of carboxylic acids
A metal-catalysed functional group metathesis approach to the carbon isotope labelling of carboxylic acids
The preparation of 14C-labelled compounds is a crucial step in pharmaceutical development but typically requires using toxic, radioactive gases. Now a broadly applicable functional group metathesis reaction has been developed that forms 14C-labelled carboxylic acids in one pot, without added gases, via dynamic exchange with an easily handled carboxylic acid 14C source.
- R. Garrison Kinney
- , José Zgheib
- & Bruce A. Arndtsen
-
Article
| Open Access Operando film-electrochemical EPR spectroscopy tracks radical intermediates in surface-immobilized catalysts
Operando film-electrochemical EPR spectroscopy tracks radical intermediates in surface-immobilized catalysts
Although surface-bound molecular catalysts offer well-defined active sites on heterogeneous supports, it is challenging to identify key radical intermediates in the reaction mechanism. Now, a characterization method has been developed that combines film electrochemistry and EPR spectroscopy to track radical intermediates in real time, exemplified by alcohol oxidation with a surface-immobilized nitroxide.
- Maryam Seif-Eddine
- , Samuel J. Cobb
- & Maxie M. Roessler
-
Article |
 Mapping the mechanisms of oxidative addition in cross-coupling reactions catalysed by phosphine-ligated Ni(0)
Mapping the mechanisms of oxidative addition in cross-coupling reactions catalysed by phosphine-ligated Ni(0)
The mechanism for the oxidative addition of aryl halides to nickel(0)–phosphine complexes was proposed over four decades ago. Now, this elementary reaction, which occurs during common cross-coupling reactions, has been re-examined. Both one- and two-electron pathways occur, and their relative contribution depends on the electronic properties of the reaction partners.
- Christina N. Pierson
- & John F. Hartwig
-
Article |
 Biocatalytic strategy for the construction of sp3-rich polycyclic compounds from directed evolution and computational modelling
Biocatalytic strategy for the construction of sp3-rich polycyclic compounds from directed evolution and computational modelling
The use of biocatalysis to support early-stage drug discovery campaigns remains largely untapped. Here, engineered biocatalysts enable the synthesis of sp3-rich polycyclic compounds through an intramolecular cyclopropanation of benzothiophenes, affording a class of complex scaffolds potentially useful for fragment-based drug discovery campaigns.
- David A. Vargas
- , Xinkun Ren
- & Rudi Fasan
-
Article
| Open Access Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Chelation enables selectivity control in enantioconvergent Suzuki–Miyaura cross-couplings on acyclic allylic systems
Achieving selectivity control in allylic arylations is a long-standing challenge in catalysis. Now a rhodium-catalysed system demonstrates chemo-, regio- and enantioselectivity, enabling Suzuki–Miyaura-type arylation with racemic, non-symmetrical, acyclic allylic systems; chelation is speculated to facilitate oxidative addition and enable both enantiomers of the starting material to converge onto a single product.
- Violeta Stojalnikova
- , Stephen J. Webster
- & Stephen P. Fletcher
-
Article |
 Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
Synthesis of polysubstituted azepanes by dearomative ring expansion of nitroarenes
While saturated N-heterocycles are widespread motifs in drug discovery, the seven-membered ring azepane is highly underrepresented. Now nitroarenes have been validated as competent substrates for azepane synthesis through conversion into singlet nitrenes for ring enlargement via N insertion and hydrogenolysis. This enables a highly versatile access towards polysubstituted azepanes in just two steps.
- Rory Mykura
- , Raquel Sánchez-Bento
- & Daniele Leonori
-
Article
| Open Access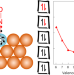 Ten-electron count rule for the binding of adsorbates on single-atom alloy catalysts
Ten-electron count rule for the binding of adsorbates on single-atom alloy catalysts
Single-atom alloys have emerged as highly active and selective catalysts that do not follow the traditional models of heterogeneous catalysis. Now it has been shown that the binding of adsorbates at their surface abides by a simple 10-electron count rule, which can identify promising catalysts for various applications.
- Julia Schumann
- , Michail Stamatakis
- & Romain Réocreux
-
Article |
 Light-driven ammonia synthesis under mild conditions using lithium hydride
Light-driven ammonia synthesis under mild conditions using lithium hydride
Photon-driven dinitrogen reduction to ammonia is a promising but challenging reaction. Now it has been shown that lithium hydride undergoes photolysis upon ultraviolet illumination to yield long-lived photon-generated electrons residing in hydrogen vacancies, favouring the activation of the N≡N bond and achieving photocatalytic ammonia synthesis.
- Yeqin Guan
- , Hong Wen
- & Ping Chen
-
Article
| Open Access Chiral arylsulfinylamides as reagents for visible light-mediated asymmetric alkene aminoarylations
Chiral arylsulfinylamides as reagents for visible light-mediated asymmetric alkene aminoarylations
Single-electron-mediated difunctionalizations of internal olefins allow the simultaneous formation of two contiguous Csp3-stereocentres. Here, we describe enantioenriched arylsulfinylamides as all-in-one reagents for the efficient asymmetric, intermolecular aminoarylation of alkenes. Mechanistic studies revealed an interesting dichotomy in the initiation of the reaction depending on the olefin redox potential.
- Cédric Hervieu
- , Mariia S. Kirillova
- & Cristina Nevado
-
Article |
 A general alkene aminoarylation enabled by N-centred radical reactivity of sulfinamides
A general alkene aminoarylation enabled by N-centred radical reactivity of sulfinamides
Single-step addition of an aryl ring and a protected amine across an alkene is a succinct route to valuable phenethylamine products, but existing methods suffer from limited scope. Now a family of compounds containing a sulfinamide functional group have been developed to react via electrophilic radicals to yield phenethylamines through an aryl migration with precise stereochemical control.
- Efrey A. Noten
- , Cody H. Ng
- & Corey R. J. Stephenson
-
News & Views |
 Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Tetracoordinate boron molecules are the key intermediates in many organoboron-related chemical transformations. Now, using alkynyl tetracoordinate boron species, a nickel-catalysed asymmetric 1,3-metallate shift towards axial chirality has been developed, giving access to various axially chiral alkenes in high efficiency.
- Li-Qing Ren
- & Chuan He
-
Article |
 Metastable gallium hydride mediates propane dehydrogenation on H2 co-feeding
Metastable gallium hydride mediates propane dehydrogenation on H2 co-feeding
The heterogeneous catalytic dehydrogenation of hydrocarbons usually suffers from a negative pressure dependence on H2. Now it has been shown that for propane dehydrogenation on gallium oxide-based catalysts, a positive activity dependence on H2 partial pressure arises from a metastable hydride-mediated catalysis in which gallium hydrides promote C–H activation.
- Guodong Sun
- , Zhi-Jian Zhao
- & Jinlong Gong
-
Article |
 Click processes orthogonal to CuAAC and SuFEx forge selectively modifiable fluorescent linkers
Click processes orthogonal to CuAAC and SuFEx forge selectively modifiable fluorescent linkers
Orthogonal click reactions enable the rapid assembly of molecules of all sizes. Now, it has been shown that a nitrile, an allene, a diborane and a hydrazine or aniline can be catalytically merged in a click process that is orthogonal to SuFEx and CuAAC, delivering fluorescent linkages.
- Paulo H. S. Paioti
- , Katherine E. Lounsbury
- & Amir H. Hoveyda
-
Article |
 Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction
Robust protocols for the synthesis of chiral α-tertiary amino acids remain scarce due to the challenge of constructing congested tetrasubstituted stereocentres. Now a cobalt-catalysed enantioselective aza-Barbier reaction of ketimines with various unactivated alkyl halides has been developed, forming diverse chiral α-tertiary amino esters with high enantioselectivity and excellent functional group tolerance.
- Xianqing Wu
- , Hanyu Xia
- & Yifeng Chen
-
Article |
 Organocatalytic aromatization-promoted umpolung reaction of imines
Organocatalytic aromatization-promoted umpolung reaction of imines
The umpolung functionalization of imines bears vast synthetic potential, but polarity inversion is less efficient compared with the carbonyl counterparts. Now, an alternative strategy exploiting chiral phosphoric acid catalytic aromatization has been developed, affording structures possessing a central chirality or a stereogenic C–N axis with high efficiency and enantiocontrol.
- Ye-Hui Chen
- , Meng Duan
- & Bin Tan
-
Article |
 A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution
A general copper-catalysed enantioconvergent C(sp3)–S cross-coupling via biomimetic radical homolytic substitution
Methods for transition-metal-catalysed enantioselective C(sp3)–S bond construction are underdeveloped. Now, by taking advantage of the biomimetic radical homolytic substitution manifold, the copper-catalysed enantioconvergent C(sp3)–S cross-coupling of racemic secondary and tertiary alkyl halides with highly transformable sulfur nucleophiles has been realized. This reaction provides access to an array of α-chiral alkyl organosulfur compounds.
- Yu Tian
- , Xi-Tao Li
- & Xin-Yuan Liu
-
Article |
 Cooperative H2 activation at a nickel(0)–olefin centre
Cooperative H2 activation at a nickel(0)–olefin centre
Activation of H2 by a metal–olefin complex is characterized experimentally and computationally using a nickel pincer complex, showing that the reaction proceeds via a direct ligand-to-ligand hydrogen transfer mechanism. An application of this cooperative H2-activation mechanism is demonstrated in the nickel-catalysed semihydrogenation of diphenylacetylene.
- María L. G. Sansores-Paredes
- , Martin Lutz
- & Marc-Etienne Moret
-
News & Views |
 From feedstock to pharmaceuticals
From feedstock to pharmaceuticals
Fluoroalkyl fragments are ubiquitous motifs in pharmaceuticals and agrochemicals, but their introduction to a given molecule typically involves expensive or difficult-to-handle reagents. Now, the photocatalysed hydrofluoroalkylation of alkenes has been achieved using simple and readily available fluoroalkyl carboxylic acids.
- Fabio Juliá
-
News & Views |
 An acid for an acid
An acid for an acid
Stereoselective decarboxylative protonation can produce diverse chiral molecules from widely available carboxylic acids. However, general and practical strategies are lacking. Now, a chiral spirocyclic phosphoric acid-catalysed decarboxylation of aminomalonic acids has enabled the modular synthesis of α-amino acids.
- Xufeng Lin
- & Alemayehu Gashaw Woldegiorgis
-
Article |
 Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer
Remote stereocontrol with azaarenes via enzymatic hydrogen atom transfer
The inherent rigidity of the azaarene ring structure has made it challenging to achieve remote stereocontrol through asymmetric catalysis on these substrates. Now, through a photoenzymatic process, an ene-reductase system facilitates the production of diverse azaarenes with distant γ-stereocentres, highlighting the potential of biocatalysts for stereoselectivity at remote sites.
- Maolin Li
- , Wesley Harrison
- & Huimin Zhao
-
Article |
 Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Modular and diverse synthesis of amino acids via asymmetric decarboxylative protonation of aminomalonic acids
Asymmetric decarboxylation can transform abundant carboxylic acids into valuable chiral molecules but faces major limitations due to the challenging enantiocontrol of proton transfer. Now the use of Brønsted acid catalysis in conjunction with an anchoring group strategy has enabled the decarboxylative protonation of aminomalonic acids to access diverse amino acids.
- Wei-Feng Zheng
- , Jingdan Chen
- & Zhongxing Huang
-
Article |
 Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids
Photocatalytic hydrofluoroalkylation of alkenes with carboxylic acids
Alkene hydrofluoroalkylation offers a promising route to diverse fluoroalkylated compounds but current methods have limitations, such as needing expensive fluoroalkylating reagents. Now, leveraging iron photocatalysis and hydrogen-atom-transfer catalysis, a hydrofluoroalkylation method has been developed that utilizes feedstock chemicals such as trifluoroacetic acid as direct fluoroalkyl radical precursors, providing a redox-neutral, general protocol to introduce fluoroalkyl moieties.
- Kang-Jie Bian
- , Yen-Chu Lu
- & Julian G. West
-
Article |
 Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
Discovery and synthesis of atropisomerically chiral acyl-substituted stable vinyl sulfoxonium ylides
The synthesis of optically enriched atropisomers has so far been limited to molecules containing aryl groups. Now a variant of non-aryl atropisomerism has been identified in vinyl sulfoxonium ylides, and an organocatalytic method has been developed to produce these molecules. This type of axial chirality is characterized by restricted rotation of the central C(sp2)–C(sp2) bond.
- Fengjin Wu
- , Yichi Zhang
- & Yong Huang
-
-
News & Views |
 Enyne difluorination
Enyne difluorination
Fluorination strategies are important in assisting the synthesis of pharmaceuticals. Iodine(I/III) catalysis has become particularly useful for installing gem-difluoro groups but is limited to styrenes. Now, the hypervalent iodane-catalysed difluorination of enynes has enabled access to diverse homopropargylic difluorides.
- Rachel C. Epplin
- & Tanja Gulder
-
Article
| Open Access Regioselective, catalytic 1,1-difluorination of enynes
Regioselective, catalytic 1,1-difluorination of enynes
Hypervalent iodine catalysis remains a powerful method to enable geminal difluoromethylenation of alkenes. However, the scope is mainly limited to styrene derivatives. Now, enynes have been validated as competent substrates where a formal 1,2-shift of the alkyne occurs, thereby enabling highly versatile homopropargylic difluorides to be generated.
- Zi-Xuan Wang
- , Keith Livingstone
- & Ryan Gilmour
-
Article |
 A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media
A crystalline aluminium–carbon-based ambiphile capable of activation and catalytic transfer of ammonia in non-aqueous media
The reversible N–H activation and catalytic transformations of ammonia are a challenge. Now, a hidden frustrated Lewis pair is shown to activate non-aqueous ammonia thermoneutrally and split the N–H bond reversibly at ambient temperature. The N–H-activated ammonia was also utilized as an atom-economical nitrogen source for catalytic NH3 transfer reactions.
- Felix Krämer
- , Jan Paradies
- & Frank Breher
-
Research Briefing |
 Iron(iii) porphyrin complexes as metalloradical catalysts
Iron(iii) porphyrin complexes as metalloradical catalysts
Experimental and computational studies establish the operation of Fe(iii)-based metalloradical catalysis for the asymmetric cyclopropanation of alkenes with different classes of diazo compounds. The reaction proceeds through a stepwise radical mechanism involving α-Fe(iv)-alkyl and γ-Fe(iv)-alkyl radical intermediates. This work provides a future direction for the development of metalloradical catalysis.
-
News & Views |
 Hydrogenative double-bond deuteration
Hydrogenative double-bond deuteration
Deuterated compounds are used in many applications such as mass-spectrometry standards, drugs or in organic light-emitting diodes. Now, hydrogen-activated homogeneous pincer complex catalysts can be used to perform selective alkene deuteration with the cheapest available deuterium source, D2O.
- Anika Tarasewicz
- & Volker Derdau
-
News & Views |
 Aryl ortho methoxylation
Aryl ortho methoxylation
Aryl ethers are useful intermediates in organic synthesis and are found in countless biologically active compounds. Now, through palladium/norbornene cooperative catalysis and incorporation of a polarity-reversed N–O reagent as the O-electrophile, an efficient arene methoxylation approach has been successfully developed.
- Kun Zhao
- & Zhenhua Gu
-
Article |
 Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism
Cobalt(II) complexes of porphyrins have dominated the development of metalloradical catalysts. Now it has been shown that five-coordinate iron(III) complexes of porphyrins with an axial ligand are also potent metalloradical catalysts for olefin cyclopropanation. They are shown to react with different classes of diazo compounds via a stepwise radical mechanism.
- Wan-Chen Cindy Lee
- , Duo-Sheng Wang
- & X. Peter Zhang
-
Article |
 Hydrogenative alkene perdeuteration aided by a transient cooperative ligand
Hydrogenative alkene perdeuteration aided by a transient cooperative ligand
Deuterogenation methods typically introduce only two deuterium atoms per unsaturation. Now the single-step hydrogenative perdeuteration of alkenes has been achieved using H2 and D2O, with incorporation of up to 4.9 D atoms per C=C double bond. The reaction is catalysed by a ruthenium pincer complex with a catalytic amount of thiol, which serves as a transient cooperative ligand.
- Jie Luo
- , Lijun Lu
- & David Milstein
-
Article |
 Ortho-C–H methoxylation of aryl halides enabled by a polarity-reversed N–O reagent
Ortho-C–H methoxylation of aryl halides enabled by a polarity-reversed N–O reagent
Pd/norbornene cooperative catalysis provides a strategy for arene functionalization, but the electrophile scope is typically limited to ‘soft’ elements such as carbon, nitrogen and sulfur. Now the ortho-C–H methoxylation of aryl halides has been realized using a polarity-reversed N−O reagent and facilitated by a C7-bromo-substituted norbornene mediator.
- Xin Liu
- , Yue Fu
- & Guangbin Dong
-
News & Views |
 Separating fiction from fact for photocatalytic CO2 reduction
Separating fiction from fact for photocatalytic CO2 reduction
Although light-driven conversion of carbon dioxide receives widespread attention, it is also criticized due to the challenge of discerning true product formation from that of impurities. Now, significantly advanced guidelines for proper product identification have been developed, so we can better trust in what we see.
- Jennifer Strunk
-
Article |
 Enhanced active-site electric field accelerates enzyme catalysis
Enhanced active-site electric field accelerates enzyme catalysis
The design and improvement of enzymes based on physical principles remain challenging. Now, the vibrational Stark effect has been used to demonstrate how an electrostatic model can unify the catalytic effects of distinct chemical forces in a quantitative manner and guide the design of enzyme variants that outperform their natural counterpart.
- Chu Zheng
- , Zhe Ji
- & Steven G. Boxer

 Modular chemoenzymatic synthesis of ten fusicoccane diterpenoids
Modular chemoenzymatic synthesis of ten fusicoccane diterpenoids Taking copper’s pulse with Raman
Taking copper’s pulse with Raman