Abstract
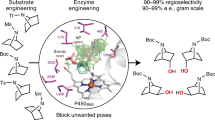
The hallmark of enzymes from secondary metabolic pathways is the pairing of powerful reactivity with exquisite site selectivity. The application of these biocatalytic tools in organic synthesis, however, remains under-utilized due to limitations in substrate scope and scalability. Here, we report how the reactivity of a monooxygenase (PikC) from the pikromycin pathway is modified through computationally guided protein and substrate engineering, and applied to the oxidation of unactivated methylene C–H bonds. Molecular dynamics and quantum mechanical calculations were used to develop a predictive model for substrate scope, site selectivity and stereoselectivity of PikC-mediated C–H oxidation. A suite of menthol derivatives was screened computationally and evaluated through in vitro reactions, where each substrate adhered to the predicted models for selectivity and conversion to product. This platform was also expanded beyond menthol-based substrates to the selective hydroxylation of a variety of substrate cores ranging from cyclic to fused bicyclic and bridged bicyclic compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Newhouse, T. & Baran, P. S. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Ed. 50, 3362–3374 (2011).
Gormisky, P. E. & White, M. C. Catalyst-controlled aliphatic C–H oxidations with a predictive model for site-selectivity. J. Am. Chem. Soc. 135, 14052–14055 (2013).
Chen, M. S. & White, M. C. Combined effects on selectivity in Fe-catalyzed methylene oxidation. Science 327, 566–571 (2010).
Chen, K., Eschenmoser, A. & Baran, P. S. Strain release in C–H bond activation. Angew. Chem. Int. Ed. 48, 9705–9708 (2009).
Roiban, G. D., Agudo, R. & Reetz, M. T. Cytochrome P450 catalyzed oxidative hydroxylation of achiral organic compounds with simultaneous creation of two chirality centers in a single C–H activation step. Angew. Chem. Int. Ed. 53, 8659–8663 (2014).
Kille, S., Zilly, F. E., Acevedo, J. P. & Reetz, M. T. Regio- and stereoselectivity of P450-catalysed hydroxylation of steroids controlled by laboratory evolution. Nature Chem. 3, 738–743 (2011).
Zhang, K. D., Shafer, B. M., Demars, M. D., Stern, H. A. & Fasan, R. Controlled oxidation of remote sp3 C–H bonds in artemisinin via P450 catalysts with fine-tuned regio- and stereoselectivity. J. Am. Chem. Soc. 134, 18695–18704 (2012).
Zhang, K. D., El Damaty, S. & Fasan, R. P450 fingerprinting method for rapid discovery of terpene hydroxylating P450 catalysts with diversified regioselectivity. J. Am. Chem. Soc. 133, 3242–3245 (2011).
Lewis, J. C., Coelho, P. S. & Arnold, F. H. Enzymatic functionalization of carbon–hydrogen bonds. Chem. Soc. Rev. 40, 2003–2021 (2011).
Fasan, R. Tuning P450 enzymes as oxidation catalysts. ACS Catal. 2, 647–666 (2012).
Agudo, R., Roiban, G. D. & Reetz, M. T. Achieving regio- and enantioselectivity of P450-catalyzed oxidative C–H activation of small functionalized molecules by structure-guided directed evolution. ChemBioChem 13, 1465–1473 (2012).
Acevedo-Rocha, C. G., Hoebenreich, S. & Reetz, M. T. in Methods in Molecular Biology 2nd edn, Vol. 1179 (eds Gillam, E. M. J., Copp, J. N. & Ackerley, D. F.) 103–128 (Humana Press, 2014).
Jung, S. T., Lauchli, R. & Arnold, F. H. Cytochrome P450: taming a wild type enzyme. Curr. Opin. Biotechnol. 22, 809–817 (2011).
Yang, J., Gabriele, B., Belvedere, S., Huang, Y. & Breslow, R. Catalytic oxidations of steroid substrates by artificial cytochrome P-450 enzymes. J. Org. Chem. 67, 5057–5067 (2002).
Breslow, R. et al. Remote oxidation of steroids by photolysis of attached benzophenone groups. J. Am. Chem. Soc. 95, 3251–3262 (1973).
Leow, D., Li, G., Mei, T. S. & Yu, J. Q. Activation of remote meta-C–H bonds assisted by an end-on template. Nature 486, 518–522 (2012).
Tang, R. Y., Li, G. & Yu, J. Q. Conformation-induced remote meta-C–H activation of amines. Nature 507, 215–220 (2014).
Larsen, A. T., May, E. M. & Auclair, K. Predictable stereoselective and chemoselective hydroxylations and epoxidations with P450 3A4. J. Am. Chem. Soc. 133, 7853–7858 (2011).
Menard, A., Fabra, C., Huang, Y. & Auclair, K. Type II ligands as chemical auxiliaries to favor enzymatic transformations by P450 2E1. ChemBioChem 13, 2527–2536 (2012).
Polic, V. & Auclair, K. Controlling substrate specificity and product regio- and stereo-selectivities of P450 enzymes without mutagenesis. Bioorg. Med. Chem. 22, 5547–5554 (2014).
Vermeulen, N. A., Chen, M. S. & White, M. C. The Fe(PDP)-catalyzed aliphatic C–H oxidation: a slow addition protocol. Tetrahedron 65, 3078–3084 (2009).
Olivo, G., Lanzalunga, O., Mandolini, L. & Di Stefano, S. Substituent effects on the catalytic activity of bipyrrolidine-based iron complexes. J. Org. Chem. 78, 11508–11512 (2013).
Yazerski, V. A. et al. Making Fe(BPBP)-catalyzed C–H and C=C oxidations more affordable. Org. Biomol. Chem. 12, 2062–2070 (2014).
Lee, S. & Fuchs, P. L. An efficient C–H oxidation protocol for alpha-hydroxylation of cyclic steroidal ethers. Org. Lett. 6, 1437–1440 (2004).
Ottenbacher, R. V., Samsonenko, D. G., Talsi, E. P. & Bryliakov, K. P. Highly efficient, regioselective, and stereospecific oxidation of aliphatic C–H groups with H2O2, catalyzed by aminopyridine manganese complexes. Org. Lett. 14, 4310–4313 (2012).
Chow, Y. L., Hayasaka, T. & Tam, J. N. S. Photoreaction of nitroso compounds in solution. 13. Photo-oxidation of alkyl nitrites. Can. J. Chem. 48, 508–511 (1970).
Petrovic, G., Saicic, R. N. & Cekovic, Z. Regioselective free radical phenylsulfenation of a non-activated delta-carbon atom by the photolysis of alkyl benzenesulfenate. Tetrahedron 59, 187–196 (2003).
Miyazawa, M., Kumagae, S. & Kameoka, H. Biotransformation of (+)- and (–)-menthol by the larvae of common cutworm (Spodoptera litura). J. Agric. Food Chem. 47, 3938–3940 (1999).
Atta ur, R. et al. Fungal transformation of (1R,2S,5R)-(–)-menthol by Cephalosporium aphidicola. J. Nat. Prod. 61, 1340–1342 (1998).
Xue, Y. Q., Zhao, L. S., Liu, H. W. & Sherman, D. H. A gene cluster for macrolide antibiotic biosynthesis in Streptomyces venezuelae: architecture of metabolic diversity. Proc. Natl Acad. Sci. USA 95, 12111–12116 (1998).
Xue, Y. Q., Wilson, D., Zhao, L. S., Liu, H. W. & Sherman, D. H. Hydroxylation of macrolactones YC-17 and narbomycin is mediated by the PikC-encoded cytochrome P450 in Streptomyces venezuelae. Chem. Biol. 5, 661–667 (1998).
Zhang, Q. B. & Sherman, D. H. Isolation and structure determination of novamethymycin, a new bioactive metabolite of the methymycin biosynthetic pathway in Streptomyces venezuelae. J. Nat. Prod. 64, 1447–1450 (2001).
Sherman, D. H. et al. The structural basis for substrate anchoring, active site selectivity, and product formation by P450 PikC from Streptomyces venezuelae. J. Biol. Chem. 281, 26289–26297 (2006).
Li, S. Y., Ouellet, H., Sherman, D. H. & Podust, L. M. Analysis of transient and catalytic desosamine-binding pockets in cytochrome P-450 PikC from Streptomyces venezuelae. J. Biol. Chem. 284, 5723–5730 (2009).
Li, S. Y. et al. Selective oxidation of carbolide C–H bonds by an engineered macrolide P450 mono-oxygenase. Proc. Natl Acad. Sci. USA 106, 18463–18468 (2009).
Negretti, S. et al. Directing group-controlled regioselectivity in an enzymatic C–H bond oxygenation. J. Am. Chem. Soc. 136, 4901–4904 (2014).
Jiménez-Osés, G. et al. The role of distant mutations and allosteric regulation on LovD active site dynamics. Nature Chem. Biol. 10, 431–436 (2014).
Li, S. Y., Podust, L. M. & Sherman, D. H. Engineering and analysis of a self-sufficient biosynthetic cytochrome P450 PikC fused to the RhFRED reductase domain. J. Am. Chem. Soc. 129, 12940–12941 (2007).
Wong, C-H. & Whitesides, G. M. Enzyme-catalyzed organic synthesis: NAD(P)H cofactor regeneration by using glucose-6-phosphate and glucose-6-phosphate dehydrogenase from Leuconostoc mesenteroides. J. Am. Chem. Soc. 103, 4890–4899 (1981).
Frisch, M. J. et al. Gaussian 09 (Gaussian, 2009).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Shaik, S. et al. P450 enzymes. Their structure, reactivity, and selectivity—modeled by QM/MM calculations. Chem. Rev. 110, 949–1017 (2009).
Rydberg, P., Sigfridsson, E. & Ryde, U. On the role of the axial ligand in heme proteins: a theoretical study. J. Biol. Inorg. Chem. 9, 203–223 (2004).
Hay, P. J. & Wadt, W. R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 82, 270–283 (1985).
Grimme, S., Ehrlich, S. & Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 32, 1456–1465 (2011).
Zhao, Y. & Truhlar, D. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 120, 215–241 (2008).
Dolg, M., Wedig, U., Stoll, H. & Preuss, H. Energy-adjusted ab initio pseudopotentials for the first row transition elements. J. Chem. Phys. 86, 866–872 (1987).
Gonzalez, C. & Schlegel, H. B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 94, 5523–5527 (1990).
Gonzalez, C. & Schlegel, H. B. An improved algorithm for reaction path following. J. Chem. Phys. 90, 2154–2161 (1989).
Gerenkamp, M. & Grimme, S. Spin-component scaled second-order Moller–Plesset perturbation theory for the calculation of molecular geometries and harmonic vibrational frequencies. Chem. Phys. Lett. 392, 229–235 (2004).
Dunning, T. H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 90, 1007–1023 (1989).
Salomon-Ferrer, R., Götz, A. W., Poole, D., Le Grand, S. & Walker, R. C. Routine microsecond molecular dynamics simulations with AMBER on Gpus. 2. Explicit solvent particle mesh ewald. J. Chem. Theory Comput. 9, 3878–3888 (2013).
Case, D. A. et al. AMBER 12 (UCSF, 2012).
Shahrokh, K., Orendt, A., Yost, G. S. & Cheatham, T. E. Quantum mechanically derived AMBER-compatible heme parameters for various states of the cytochrome P450 catalytic cycle. J. Comput. Chem. 33, 119–133 (2012).
Wang, J. M., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general Amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Bayly, C. I., Cieplak, P., Cornell, W. D. & Kollman, P. A. A Well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Besler, B. H., Merz, K. M. & Kollman, P. A. Atomic charges derived from semiempirical methods. J. Comput. Chem. 11, 431–439 (1990).
Singh, U. C. & Kollman, P. A. An approach to computing electrostatic charges for molecules. J. Comput. Chem. 5, 129–145 (1984).
Jorgensen, W. L., Chandrasekhar, J., Madura, J. D., Impey, R. W. & Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 79, 926–935 (1983).
Wang, J. M., Cieplak, P. & Kollman, P. A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 21, 1049–1074 (2000).
Darden, T., York, D. & Pedersen, L. Particle mesh Ewald: an N-log(N) method for Ewald sums in large systems. J. Chem. Phys. 98, 10089–10092 (1993).
Acknowledgements
This work was supported by the National Science Foundation under the CCI Center for Selective C−H Functionalization (CHE-1205646) and the National Institutes of Health (NIH R01 grant GM078553 to D.H.S., J.M. and L.M.P. and grant GM075962 to K.N.H.). Calculations were performed using the Extreme Science and Engineering Discovery Environment (XSEDE), which is funded by the NSF (OCI-1053575), and the UCLA Institute of Digital Research and Education (IDRE). The authors also acknowledge the Life Sciences Research Foundation for a postdoctoral fellowship (to A.R.H.N.) and the University of Michigan Chemistry–Biology Interface (CBI) training programme (GM008597, to S.N.). J. Stachowski is thanked for discussions.
Author information
Authors and Affiliations
Contributions
A.R.H.N. and G.J-O. contributed equally to this work. A.R.H.N., G.J-O., P.L., S.N., J.M., K.N.H. and D.H.S. conceived, designed and supervised the project. G.J-O., P.L., R.O.R., Y-F.Y., L.R.F. and Z.L. performed the computational experiments. A.R.H.N., S.N., W.Z. and M.M.G. synthesized substrates and characterized products. A.R.H.N. conducted the biochemical experiments. A.R.H.N., G.J-O., P.L., L.M.P., J.M., K.N.H. and D.H.S. wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 10898 kb)
Rights and permissions
About this article
Cite this article
Narayan, A., Jiménez-Osés, G., Liu, P. et al. Enzymatic hydroxylation of an unactivated methylene C–H bond guided by molecular dynamics simulations. Nature Chem 7, 653–660 (2015). https://doi.org/10.1038/nchem.2285
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2285
This article is cited by
-
Unnatural activities and mechanistic insights of cytochrome P450 PikC gained from site-specific mutagenesis by non-canonical amino acids
Nature Communications (2023)
-
Iridium and B(C6F5)3 co-catalyzed chemoselective deoxygenative reduction of tertiary amides: application to the efficient synthesis and late-stage modification of pharmaceuticals
Science China Chemistry (2023)
-
Enantioselective oxidation of unactivated C–H bonds in cyclic amines by iterative docking-guided mutagenesis of P450BM3 (CYP102A1)
Nature Synthesis (2022)
-
Pervasive cooperative mutational effects on multiple catalytic enzyme traits emerge via long-range conformational dynamics
Nature Communications (2021)
-
Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models
Nature Chemistry (2016)