Abstract
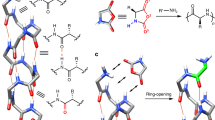
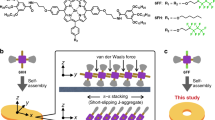
Catalysis observed in enzymatic processes and protein polymerizations often relies on the use of supramolecular interactions and the organization of functional elements in order to gain control over the spatial and temporal elements of fundamental cellular processes. Harnessing these cooperative interactions to catalyse reactions in synthetic systems, however, remains challenging due to the difficulty in creating structurally controlled macromolecules. Here, we report a polypeptide-based macromolecule with spatially organized α-helices that can catalyse its own formation. The system consists of a linear polymeric scaffold containing a high density of initiating groups from which polypeptides are grown, forming a brush polymer. The folding of polypeptide side chains into α-helices dramatically enhances the polymerization rate due to cooperative interactions of macrodipoles between neighbouring α-helices. The parameters that affect the rate are elucidated by a two-stage kinetic model using principles from nucleation-controlled protein polymerizations; the key difference being the irreversible nature of this polymerization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Breslow, R. Biomimetic chemistry and artificial enzymes: catalysis by design. Acc. Chem. Res. 28, 146–153 (1995).
Dong, Z., Luo, Q. & Liu, J . Artificial enzymes based on supramolecular scaffolds. Chem. Soc. Rev. 41, 7890–7908 (2012).
Kuah, E., Toh, S., Yee, J., Ma, Q. & Gao, Z . Enzyme mimics: advances and applications. Chem. Eur. J. 22, 8404–8430 (2016).
Lund, K. et al. Molecular robots guided by prescriptive landscapes. Nature 465, 206–210 (2010).
Wickham, S. F. J. et al. A DNA-based molecular motor that can navigate a network of tracks. Nat. Nanotechnol. 7, 169–173 (2012).
Erbas-Cakmak, S., Leigh, D. A., McTernan, C. T. & Nussbaumer, A. L. Artificial molecular machines. Chem. Rev. 115, 10081–10206 (2015).
Browne, W. R. & Feringa, B. L . Making molecular machines work. Nat. Nanotechnol. 1, 25–35 (2006).
van Dongen, S. F. M., Cantekin, S., Elemans, J. A. A. W., Rowan, A. E. & Nolte, R. J. M. Functional interlocked systems. Chem. Soc. Rev. 43, 99–122 (2014).
Epstein, I. R. & Xu, B. Reaction-diffusion processes at the nano- and microscales. Nat. Nanotechnol. 11, 312–319 (2016).
Boekhoven, J., Hendriksen, W. E., Koper, G. J. M., Eelkema, R. & van Esch, J. H. Transient assembly of active materials fueled by a chemical reaction. Science 349, 1075–1079 (2015).
Sadownik, J. W., Mattia, E., Nowak, P. & Otto, S. Diversification of self-replicating molecules. Nat. Chem. 8, 264–269 (2016).
Lewandowski, B. et al. Sequence-specific peptide synthesis by an artificial small-molecule machine. Science 339, 189–193 (2013).
Korevaar, P. A. et al. Pathway complexity in supramolecular polymerization. Nature 481, 492–496 (2012).
Aliprandi, A., Mauro, M. & De Cola, L. Controlling and imaging biomimetic self-assembly. Nat. Chem. 8, 10–15 (2016).
Korevaar, P. A., Newcomb, C. J., Meijer, E. W. & Stupp, S. I. Pathway selection in peptide amphiphile assembly. J. Am. Chem. Soc. 136, 8540–8543 (2014).
Yu, Z. et al. Simultaneous covalent and noncovalent hybrid polymerizations. Science 351, 497–502 (2016).
Zhao, D. & Moore, J. S. Nucleation-elongation: a mechanism for cooperative supramolecular polymerization. Org. Biomol. Chem. 1, 3471–3491 (2003).
Oosawa, F. & Asakura, S. Thermodynamics of the Polymerization of Protein (Academic Press, 1975).
Luders, J. & Stearns, T. Microtubule-organizing centres: a re-evaluation. Nat. Rev. Mol. Cell Biol. 8, 161–167 (2007).
Dominguez, R. & Holmes, K. C . Actin structure and function. Annu. Rev. Biophys. 40, 169–186 (2011).
De Greef, T. F. A. et al. Supramolecular polymerization. Chem. Rev. 109, 5687–5754 (2009).
McHale, R., Patterson, J. P., Zetterlund, P. B. & O'Reilly, R. K. Biomimetic radical polymerization via cooperative assembly of segregating templates. Nat. Chem. 4, 491–497 (2012).
Lu, H., Wang, J., Lin, Y. & Cheng, J. One-pot synthesis of brush-like polymers via integrated ring-opening metathesis polymerization and polymerization of amino acid N- carboxyanhydrides. J. Am. Chem. Soc. 131, 13582–13583 (2009).
Lu, H. & Cheng, J. N-Trimethylsilyl amines for controlled ring-opening polymerization of amino acid N-carboxyanhydrides and facile end froup functionalization of polypeptides. J. Am. Chem. Soc. 130, 12562–12563 (2008).
Goodman, M., Verdini, A. S., Toniolo, C., Phillips, W. D. & Bovey, F. A. Sensitive criteria for the critical size for helix formation in oligopeptides. Proc. Natl Acad. Sci. USA 64, 444–450 (1969).
Rinaudo, M. & Domard, A. Circular dichroism studies on α-L-glutamic acid oligomers in solution. J. Am. Chem. Soc. 98, 6360–6364 (1976).
Mutter, M. The influence of the macromolecular protecting group in conformational studies on polyoxyethylene-bound peptides. Macromolecules 10, 1413–1414 (1977).
Ballard, D. G. H. & Bamford, C. H. The heterogeneous polymerization of α-N-carboxyamino-acid anhydrides. J. Chem. Soc. 1039–1044 (1959).
Kōmoto, T., Akaishi, T., Ōya, M. & Kawai, T. Crystallization of polypeptides in the course of polymerization. I.: Growth mechanism of poly-L- and DL-alanine crystals. Makromol. Chem. 154, 151–159 (1972).
Hadjichristidis, N., Iatrou, H., Pitsikalis, M. & Sakellariou, G. Synthesis of well-defined polypeptide-based materials via the ring-opening polymerization of α-amino acid N-carboxyanhydrides. Chem. Rev. 109, 5528–5578 (2009).
Deming, T. J. Polypeptide and Polypeptide Hybrid Copolymer Synthesis via NCA Polymerization (Springer, 2006).
Blout, E. R. & Idelson, M. Polypeptides IX. the kinetics of strong-base initiated polymerizations of amino acid-N-carboxyanhydrides. J. Am. Chem. Soc. 78, 3857–3858 (1956).
Lu, H. & Cheng, J. Hexamethyldisilazane-mediated controlled polymerization of α-amino acid N-carboxyanhydrides. J. Am. Chem. Soc. 129, 14114–14115 (2007).
Lundberg, R. D. & Doty, P. Polypeptides XVII. a study of the kinetics of the primary amine- initiated polymerization of N-carboxy-anhydrides with special reference to configurational and stereochemical effects. J. Am. Chem. Soc. 79, 3961–3972 (1957).
Blout, E. R. & Asadourian, A. Polypeptides. V. the infrared spectra of polypeptides derived from γ-benzyl-L-glutamate. J. Am. Chem. Soc. 78, 955–961 (1956).
Ling, J. & Huang, Y. Understanding the ring-opening reaction of α-amino acid N-carboxyanhydride in an amine-mediated living polymerization: a DFT study. Macromol. Chem. Phys. 211, 1708–1711 (2010).
Kricheldorf, H. R. α-Aminoacid-N-Carboxy-Anhydrides and Related Heterocycles (Springer, 1987).
Weingarten, H. Kinetics and mechanisms of the polymerization of N-carboxy-α-amino acid anhydrides. J. Am. Chem. Soc. 80, 352–355 (1958).
Kelly, D. R. & Roberts, S. M . The mechanism of polyleucine catalysed asymmetric epoxidation. Chem. Commun. 2018–2020 (2004).
Mathew, S. P., Gunathilagan, S., Roberts, S. M. & Blackmond, D. G. Mechanistic insights from reaction progress kinetic analysis of the polypeptide-catalyzed epoxidation of chalcone. Org. Lett. 7, 4847–4850 (2005).
Aragonès, A. C. et al. Electrostatic catalysis of a Diels–Alder reaction. Nature 531, 88–91 (2016).
Yu, M., Nowak, A. P., Deming, T. J. & Pochan, D. J. Methylated mono- and diethyleneglycol functionalized polylysines: nonionic, α-helical, water-soluble polypeptides. J. Am. Chem. Soc. 121, 12210–12211 (1999).
Rzayev, J. Synthesis of polystyrene−polylactide bottlebrush block copolymers and their melt self-assembly into large domain nanostructures. Macromolecules 42, 2135–2141 (2009).
Neugebauer, D., Sumerlin, B. S., Matyjaszewski, K., Goodhart, B. & Sheiko, S. S . How dense are cylindrical brushes grafted from a multifunctional macroinitiator? Polymer 45, 8173–8179 (2004).
Acknowledgements
The research was supported by the US National Science Foundation (CHE-1308485 and CHE-1508710 to J.C. & DMR-1150742 to Y.L.). AFM was carried out in part in the Frederick Seitz Materials Research Laboratory Central Research Facilities, University of Illinois.
Author information
Authors and Affiliations
Contributions
R.B., J.C. and Y.L. conceived the idea of the project. R.B. and Z.S. performed the experimental work. Y.L. and H.F. performed the kinetic modelling. R.B., Y.L., H.F., and J.C. wrote the manuscript with contributions from all authors. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 4886 kb)
Rights and permissions
About this article
Cite this article
Baumgartner, R., Fu, H., Song, Z. et al. Cooperative polymerization of α-helices induced by macromolecular architecture. Nature Chem 9, 614–622 (2017). https://doi.org/10.1038/nchem.2712
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2712
This article is cited by
-
Site-selected in situ polymerization for living cell surface engineering
Nature Communications (2023)
-
A nanoscale MOF-based heterogeneous catalytic system for the polymerization of N-carboxyanhydrides enables direct routes toward both polypeptides and related hybrid materials
Nature Communications (2023)
-
Stride Strategy to Enable a Quasi-ergodic Search of Reaction Pathways Demonstrated by Ring-opening Polymerization of Cyclic Esters
Chinese Journal of Polymer Science (2023)
-
Helical Nonfouling Polypeptides for Biomedical Applications
Chinese Journal of Polymer Science (2022)
-
Accelerated polymerization of N-carboxyanhydrides catalyzed by crown ether
Nature Communications (2021)