Abstract
Recently, materials revealing the upconversion (UC) phenomenon, which is a conversion of low-energy photons to higher-energy ones, have attracted considerable attention in luminescence thermometry due to the possibility of precise and remote optical thermal sensing. The most widely studied type of luminescent thermometry uses a ratiometric approach based on changes in the UC luminescence intensity, mainly of lanthanide ions’ thermally coupled energy levels. In this work, NaYF4:Ho3+@NaYF4, and NaYF4:Ho3+, Er3+@NaYF4 nanoparticles (NPs) were synthesized by the controlled reaction in oleic acid and octadecene at 573 K. The obtained nanoparticles had hexagonal structures, oval shapes, and average sizes of 22.5 ± 2.2 nm and 22.2 ± 2.0 nm, respectively. The spectroscopic properties of the products were investigated by measurements of the UC emission under 1151 nm laser excitation in the temperature range between 295 to 378 K. The sample doped with Ho3+ and Er3+ ions showed unique behavior of enhancing emission intensity with the temperature. The relative sensitivity determined for the NPs containing Ho3+ and Er3+ ions, reached the maximum value of 1.80%/K at 378 K. Here, we prove that the NaYF4:Ho3+, Er3+@NaYF4 system presents unique and excellent optical temperature sensing properties based on the luminescence intensity ratios of the near-infrared bands of both Ho3+ and Er3+ ions.
Similar content being viewed by others
Introduction
The rapid development of technology would be impossible without discoveries and inventions in multiple fields of science. The crucial aspect of various scientific studies is determining the properties of the research objects. The particularly significant parameter is temperature, which is a physical quantity important for all biological processes. Thus, temperature measurements are widely used in medicine, including early tumor detection, monitoring of brain activity, or in vivo inflammation detection1,2,3,4,5. In addition, temperature sensors are also relevant in other areas, e.g., for diagnostics of constructions, electrical installations, or even food production6,7,8,9.
However, it is difficult to verify the actual temperature of the material through a typical contact approach due to the interference from the introduced thermometers10. Thus, it is important to find a non-contact system to determine the internal temperature of the object11,12,13. The appealing method for remote temperature detection is luminescence thermometry, based on changes in the emission character of previously excited material14. The ability to prepare nanoparticles (NPs), resulting from nanotechnology development, is directly responsible for the remarkable advancements in optical nanothermometry15. The NPs’ luminescence highly depends on temperature, so the remote thermal readout is possible through a simple spectroscopic analysis16. One of the most widely studied types of optical nanothermometry is the ratiometric approach, which uses changes in the luminescence intensity17. However, to measure temperature with this method, the sensor must show at least two emission peaks whose changes in the intensities are interdependent.
The promising candidates for non-contact optical nanothermometry seem to be upconverting nanoparticles (UCNPs)18. Upconversion is a process of converting low-energy photons to high-energy ones, usually from the near-infrared (NIR) to the ultraviolet or visible range19. This phenomenon occurs for materials doped with lanthanide ions (Ln3+), which have a 4f. electronic structure with many well-defined energy levels. The unique properties of the UCNPs containing Ln3+ ions result in narrow absorption and emission bands, a significant difference between the absorption and the emission wavelengths, and relatively long luminescence lifetimes20,21,22,23. The UCNPs can be excited by NIR radiation, which benefits medical applications. Excitation of UCNPs by NIR light results in the low autofluorescence of biological materials, a high signal-to-noise ratio, and negligible photobleaching due to the low-energy excitation inorganic structure of NPs24.
Until now, the prevailing types of optical nanothermometers based on UCNPs ions have relied on the temperature-dependent emission from thermally coupled energy levels25. The UCNPs with an admixture of Er3+ ions are of great interest in this area3,26. Researchers frequently employ the thermally-coupled transitions of Er3+ ions, specifically from the excited energy levels of 2H11/2 and 4S3/2 to the ground energy level of 4I15/2. Plenty of these studies concern materials doped with Er3+ and Yb3+ ions, excited by 980 nm radiation, in which the sensitizing Yb3+ ions transfer the absorbed energy to the emitting Er3+ ions2,27,28,29. However, researchers have also published findings on UCNPs that are solely doped with Er3+ and directly excited by radiation at 980 nm or 1500 nm30,31. Moreover, a single study reported the temperature sensing properties of Er3+ doped NPs, where the luminescence originating from non-thermally coupled bands in the NIR range, specifically 4I9/2 → 4I15/2 and 4I11/2 → 4I15/2, was utilized for temperature determination31. There are also some studies on the optical thermometry of the materials emitting from the thermally or non-thermally coupled states of the other Ln3+ ions, e.g., Ho3+32,33,34, or Tm3+ ions35,36, but these materials are in general excited at 980 nm because of co-doping with Yb3+ ions.
Using Ln3+ ions other than Yb3+ in the proper matrix can lead to the UCNPs with properties promising for optical nanothermometry. For instance, a system in which Ho3+ ions act as sensitizers and Er3+ ions as emission centers can be excited in the NIR range with four different wavelengths (around 755, 900, 1150, and 1950 nm)20,37,38. The observed luminescence of these UCNPs displays transition bands originating from both Er3+ and Ho3+ ions. Depending on the temperature, alterations in the emission intensity of the individual peaks of UCNPs exhibit distinct behavior. This characteristic holds great potential for enhancing temperature sensors.
This work presents a new approach to optical nanothermometry based on the properties of Ho3+ and Er3+ ions. We selected β-sodium-yttrium fluoride as a matrix for Ln3+ ions because β-NaYF4 is an excellent UC host material with low phonon energy (around 360 cm−1)39, reducing the multiphoton quenching processes. In addition, β-NaYF4, under the proper synthesis conditions, forms crystalline NPs of small sizes with narrow size distribution. The spectroscopic properties of the prepared NaYF4:Ho3+@NaYF4, and NaYF4:Ho3+, Er3+@NaYF4 samples were measured under 1151 nm excitation in the temperature range from 295 to 378 K. The obtained results allowed us to determine the sensitivity of this nanothermometer. Our results show that a different way of excitation than the typical one at 980 nm through Yb3+ ions can also result in a good-quality optical thermometry sensor. Moreover, the used approach allows for excitation within the second biological window, which is very convenient for biomedical applications40.
Methods
Materials
Yttrium, holmium, and erbium chloride hydrates (99.99%, Alfa Aesar), sodium oleate (≥ 82%, Sigma Aldrich), and ammonium fluoride (≥ 98%, Fluka) were used as a source of Y3+, Ho3+, Er3+, Na+ and F- ions, respectively. The chlorides were placed in the dryer at 348 K for a week to remove the water (the residual water content was determined by TGA analysis). The reaction was carried out in n-octadecene (90% Alfa Aesar) and oleic acid (70% Fisher Chemicals). Ethanol (99.8% POCh S.A.) and n-hexane (≥ 99% POCh S.A.) were used to purify the post-reaction products.
NaYF4:7.5%Ho3+,7.5%Er3+@NaYF4 preparation:
-
β-core
To obtain 5.5 mmol of β-NaYF4:7.5%Ho3+,7.5%Er NPs, 110 mL of n-octadecene and oleic acid mixture (1:1), 4.6750 mmol of yttrium chloride, 0.4125 mmol of both holmium and erbium chlorides were purified at 373 K under vacuum for 2.5 h. 11.0 mmol of sodium oleate (2× excess) and 33.0 mmol of ammonium fluoride (1.5× excess) were separately added to the heated mixture under nitrogen flow and purified at 373 K under vacuum for 30 and 5 min, respectively. The mixture was heated at 573 K with vigorous stirring, under nitrogen flow for 1 h, and cooled down. The post-reaction product was purified five times by sequential dispersing in n-hexane and precipitating by ethanol (5 min, 8000 rpm). The obtained NPs were dispersed in n-hexane and air-dried for 24 h.
-
α-shell
To obtain 15 mmol of α-NaYF4 NPs, 300 mL of n-octadecene and oleic acid mixture (1:1) and 15 mmol of yttrium chloride were purified at 373 K under vacuum for 3 h. 22.5 mmol of sodium oleate (1.5× excess) and 60.0 mmol of ammonium fluoride were separately added to the heated mixture under nitrogen flow and purified at 373 K under vacuum for 45 and 10 min, respectively. The mixture was heated at 473 K with vigorous stirring, under nitrogen flow for 1 h, and cooled down. The post-reaction mixture was centrifuged (10 min, 9000 rpm), the product was precipitated by adding ethanol and purified three times by sequential disperse in n-hexane and precipitated by ethanol (5 min, 8000 rpm). The obtained NPs were air-dried for 36 h.
-
β-core@β-shell
To obtain β-NaYF4:7.5%Ho3+,7.5%Er3+@β-NaYF4 (briefly NaYF4:Ho3+, Er3+@NaYF4) NPs, 1 mmol of β-NaYF4:7.5%Ho3+,7.5%Er3+ NPs and 7 mmol of α-NaYF4 NPs were added to 32 mL of n-octadecene and oleic acid mixture (1:1) and purified at 373 K under vacuum for 3 h. Then the mixture was heated at 573 K with vigorous stirring, under nitrogen flow for 2 h and 15 min, and cooled down. The post-reaction product was purified four times by sequential dispersing in n-hexane and precipitating by ethanol (5 min, 8000 rpm). The obtained NPs were dispersed in n-hexane or air-dried for 48 h.
NaYF4:7.5%Ho3+@NaYF4 preparation:
The β-NaYF4:7.5%Ho3+@β-NaYF4 (briefly NaYF4:Ho3+@NaYF4) NPs were prepared via the above-mentioned multi-stage procedure under similar synthesis conditions. 5.0875 mmol of yttrium chloride and 0.4125 mmol of holmium chloride were used for the core preparation. The other reagents were added in the same amounts. The previously prepared α-NaYF4 NPs were also used for the NaYF4:Ho3+@NaYF4 synthesis.
Characterization
The purity of the products obtained at individual synthesis stages was determined by Thermogravimetric Analysis (TGA) on Netzsch TG 209 Libra in the temperature range from 298 to 878 K under nitrogen flow (see Fig. S1). The crystalline structures and phase purity of the prepared samples were specified by X-ray Powder Diffraction (XRD) measurements on a Bruker AXS D8 Advance Diffractometer equipped with a Johansson monochromator (λCu Kα1 = 1.5406 Å) and a LynxEye strip detector (step: 0.05° 2θ, step time: 1 s, angular range: 20–100° 2θ). The reference data was taken from JCPDS (00-016-0334). The images of synthesized NPs, based on which the average sizes and size distributions were determined, were recorded on the high-resolution transmission electron microscope Hitachi HT7700 with an accelerating voltage of 120 kV.
Measurements from 295 to 378 K were carried out in a tubular electric furnace (Gero RES-E 230/3), where the sample temperature was controlled via a type K thermocouple in contact with it. The temperature-dependent UC emission spectra of the NaYF4:Ho3+@NaYF4, and NaYF4:Ho3+, Er3+@NaYF4 NPs were obtained using a 10 ns pulsed optical parametric oscillator OPO (EKSPLA/NT342/3/UVE) as the laser source with energy 0.5 mJ. Emissions from the oven were focused on the entrance slit of a spectrograph (Andor SR-303i-A) equipped with a cooled CCD camera (Andor Newton). All spectra were corrected from the spectral response of the equipment. The QuantaMasterTM 40 spectrophotometer equipped with an Opolette 355LD UVDM tunable laser (with a repetition rate of 20 Hz) and a PIXIS:256E digital CCD camera with an SP-2156 imaging spectrograph (Princeton Instruments) was used to measure the dependencies of the energy transitions intensities on the laser energy. The luminescence rise and decay lifetimes were recorded with a Mixed Domain Oscilloscope—200 MHz—Tektronix MDO3022. These measurements were carried out for solid samples at room temperature.
Results and discussion
Structural and morphological properties
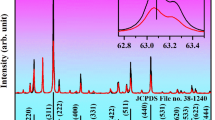
The prepared NaYF4:Ho3+@NaYF4, and NaYF4:Ho3+, Er3+@NaYF4 NPs crystallized as a single hexagonal phase, with the \(\mathrm{P}\overline{6 }\) space group (Fig. 1a). The diffraction peaks of the obtained structures align well with the reference patterns (JCPDS 00-016-0334). No significant shifts in the registered diffractograms were observed because the substitution of Y3+ ions with Ho3+ or Er3+ ions did not affect the unit cell parameters, as all ions are of similar size (\({\mathrm{r}}_{{\mathrm{Y}}^{3+}}\) = 1.040 Å, \({\mathrm{r}}_{{\mathrm{Ho}}^{3+}}\) = 1.041 Å, \({\mathrm{r}}_{{\mathrm{Er}}^{3+}}\) = 1.030 Å, see Table S1)41. The samples were characterized by small sizes, around 21 nm (by Scherrer equation), as evidenced by broad peaks in the measured diffractograms. The obtained results agree with the TEM images in Fig. 1b,c.
The obtained NPs had oval shapes and average sizes of 22.2/22.5 nm (calculated based on the TEM results) with narrow size distributions (Fig. 1b,c). In the TEM images, a slightly darker region in the centers of the NPs is visible due to about two times higher densities of the Ho3+ and Er3+ ions added to the core compared to Y3+ ions in the shell. Ultimately, the presence of the core@shell structure of the prepared samples was confirmed by the observed increase in their sizes compared with the core-only NPs (see also Fig. S2).
Spectroscopic properties
The spectroscopic studies of the prepared NPs doped with Ho3+ only, as well as Ho3+ and Er3+ ions, revealed inquire and not entirely apparent results. The excitation spectrum (Fig. S3) showed the NIR band in the range from 1130 to 1200 nm, related to the 5I8 → 5I6 Ho3+ ions energy transition20. Based on the recorded spectrum, we selected an 1151 nm laser line to measure the luminescence of the prepared samples. The excitation of the NaYF4:Ho3+@NaYF4 NPs resulted in the UC emission at 489, 544, 648, 752, 898, and 970 nm, connected with the 5F3 → 5I8; 5S2,5F4 → 5I8; 5F5 → 5I8; 5I4 → 5I8; 5I5 → 5I8 and 5F5 → 5I7 Ho3+ ions energy transitions, respectively (Fig. 2a)20. The same irradiation of the NaYF4:Ho3+, Er3+@NaYF4 NPs revealed the additional luminescence peaks of the Er3+ ions. Thus, the bands at around 523, 672, and 982 nm resulted in the 2H9/2,4S3/2 → 4I15/2; 4F9/2 → 4I15/2 and 4I11/2 → 4I15/2 Er3+ ions transitions, respectively, (also Fig. 2a)20. The sample containing only Ho3+ ions had red emission, while the emission of the Ho3+ and Er3+ ions doped sample was yellowish-orange (see the CIE chromaticity diagrams in Fig. 2b).
The UC luminescence spectra of the prepared NPs were measured under 1151 nm excitation in the temperature range from 295 to 378 K (Fig. 3). When the temperature increased, the emission intensity of the NaYF4:Ho3+@NaYF4 sample decreased. Such behavior is consistent with the general tendency of thermal quenching of luminescence due to the intensified non-radiative relaxation processes11,17. In contrast, the emission intensity of the NaYF4:Ho3+, Er3+@NaYF4 NPs increased with increasing temperature. The luminescence of this sample resulted from certain energy transfers from Ho3+ ions to Er3+ ions, so the increase of their efficiency with increasing temperature could cause the enhancement of their emission. It is worth noting that the thermal increase of Er3+ ions' luminescence intensity, particularly visible at 982 nm, was more significant than the increase of the emission intensity from only Ho3+ ions (752, 898 nm).
The parameters characterizing prepared optical temperature sensors were determined using the ratiometric approach. To describe the properties of the NaYF4:Ho3+@NaYF4 sample, the luminescence intensity ratio (LIR), which is a ratio of the luminescence intensity from the upper (IU) and lower (IL) states, was used4,42,43:
The luminescence spectra of the NaYF4:Ho3+, Er3+@NaYF4 sample, resulted from the overlapped emission from the energy levels of both Ho3+ and Er3+ ions. In that case, the LIRs were calculated as a ratio of the shorter wavelength peaks’ intensities (Is) to the intensities of the peaks with the longer wavelength (Il):
The most important parameter related to temperature-dependent luminescence, especially for the application in optical temperature sensors, is the relative sensitivity (SR) of the material to the temperature changes, determined as the rate of LIR changes with the temperature4,43,44:
The SR curves were plotted based on the LIRs’ temperature changes of the selected luminescence bands of both Ho3+ and Er3+ ions (NaYF4:Ho3+, Er3+@NaYF4 NPs) or only Ho3+ ions (NaYF4:Ho3+@NaYF4 NPs).
The LIR and SR dependencies were estimated for the NPs’ luminescence peaks with the different temperature behaviors and are shown in Fig. 4. In the case of the sample doped solely with Ho3+ ions, we selected the emission peaks at 489 and 544 nm, 648 and 898 nm, as well as 898 and 970 nm (Fig. 4a,c). When the Ho3+ and Er3+ ions were dopants, we also took into account the additional peaks that occurred at similar wavelengths: 489 and 523 + 544 nm, 648 + 672 and 898 nm, as well as 898 and 970 + 982 nm (Fig. 4b,d).
The NaYF4:Ho3+, Er3+@NaYF4 sample presented relatively high SR values with a maximum equal to 1.80 (378 K)%/K for the NIR peaks at 898 and 970 + 982 nm. In contrast, all determined SR values of the NaYF4:Ho3+@NaYF4 sample were below 1.00%/K. The results indicate that the co-doping with Er3+ ions to a system based on the Ho3+ ions significantly improves the temperature sensing properties, particularly in the NIR range. The prepared NPs exhibit minimum temperature uncertainty around 1.08 K (Fig. S4).
Determining the number of photons involved in populating the excited states of the emitting ions is crucial for explaining the mechanism behind the observed spectroscopic properties of the NPs. The photon's numbers (further described as n coefficients) are determined from the dependencies of the luminescence intensities IUC on the excitation power densities P, or in the case of the pulsed laser excitation, from its energies E45:
Luminescence peaks recorded at similar wavelengths showed significant differences in the n coefficient values between both samples (Figs. 5 and S4). The NaYF4:Ho3+@NaYF4 NPs had n values mostly between 2.0 and 3.0, which implied that mainly 3 photons are needed to obtain UC emission of Ho3+ ions. Only the emission at 544 nm was related to the absorption of 4 photons (n = 3.29). The n coefficients for the NaYF4:Ho3+, Er3+@NaYF4 NPs were mainly below 2.0, suggesting that the observed UC was primarily influenced by processes that necessitated the absorption of only 2 photons. The exception was green emission, which resulted from 3 photons process (n = 2.14).
To fully understand the nature of the observed UC phenomenon, we measured the luminescence lifetimes of the selected Ho3+ and Er3+ transitions (Fig. 6) and used the following equations to calculate average (effective) rise and decay times46:
where teff is the effective rise (R) or decay (D) time, tp is the time when the lifetime trend changes from rise to decay and I is the intensity at time t (see insets in Fig. 6).
The UC luminescence of the NaYF4:Ho3+@NaYF4 NPs results from energy transfer (ET) processes between Ho3+ ions, as evidenced by the visible rise times of the registered transitions (Fig. 6a). Moreover, the similar rise times of the transitions responsible for the 1250 and 752 nm emission (tD values around 20 μs) indicate that the 5I6 and 5I4 Ho3+ levels are likewise populated. The 5F5, 5S2, and 5F3 states related to the emission at 648, 544, and 489 nm are also populated similarly, slower than the previously mentioned, as evidenced by the congruous rise times close to 40 μs. In the case of the NaYF4:Ho3+, Er3+@NaYF4 sample, the UC luminescence at 1250 nm results from quick processes since the rise time of the 5I6 → 5I8 transition is not visible. The other emissions, which have rise times below 20 μs, are connected with the energy transfer processes between Ho3+ and Er3+ ions. In the recorded lifetime profiles of the Ho3+ ion transitions, the Er3+ ion influence is visible, especially for the red emission (see Fig. 6b).
The results of the spectroscopic measurements of the prepared NPs became the basis for the proposed mechanism responsible for the observed UC emission under 1151 nm excitation (Fig. 7).
The irradiation of the NaYF4:Ho3+@NaYF4 NPs with the 1151 nm pulsed laser produces Ho3+ ions in their 5I6 excited state via ground state absorption (GSA) process (Fig. 7a). The initially excited Ho3+ ions exchange energy with each other by the ET processes. The absorption of the subsequent photon leads to the population of the Ho3+ ions into the 5I4 levels and the weak emission at 752 nm. From this state, there is also relaxation to the 5I5 state, from which the emission at 898 nm occurs. Simultaneously, the absorption of another photon by the ET process produces the Ho3+ ions in their 5F5 state. The emission at 648 nm (5F5 → 5I8) and 970 nm (5F5 → 5I7) is from this energy level. The Ho3+ ions, previously populated to the 5I4 level, absorb another photon by the quick ESA process, whereby the Ho3+ ions are excited to their 5F3 energy levels. Hence, the sample presented emissions at 489 nm and after relaxation at 544 nm.
In the case of the NaYF4:Ho3+, Er3+@NaYF4 NPs, most of the energy absorbed by Ho3+ ions is transferred to Er3+ ions (see Fig. 7b). The Er3+ ions are excited to their 4F9/2 levels from where an additional emission is possible giving a peak at 672 nm. Further, intense emission at 982 nm occurs after the relaxation to the 4I11/2 state. Another photon absorbed within the Er3+ ion leads to a population of the 2H11/2, 4S3/2 Er3+ ions levels and an additional emission band at 523 nm. The emission of Ho3+ ions at 890 and 743 nm results from energy back transfer from the excited Er3+ ions and is weaker than in the case of a system containing only Ho3+ ions47,48. The emission of Ho3+ ions in NaYF4:Ho3+, Er3+@NaYF4 sample probably also consists of the processes occurring in sample NaYF4:Ho3+@NaYF4 sample, however, they are less intense because the pre-excited Ho3+ ions transferred most of the energy to Er3+ ions.
Conclusions
Using the precipitation reaction in the oleic acid/octadecene solution, we successfully obtained core@shell UCNPs based on sodium yttrium fluorides doped with either Ho3+ ions or both Ho3+ and Er3+ ions. The prepared UCNPs exhibited an oval shape and average sizes of approximately 22.5 nm.
The NaYF4:Ho3+@NaYF4 and NaYF4:Ho3+, Er3+@NaYF4 samples showed UC emission under 1151 nm pulsed laser excitation. We registered the emission of the products in the 295 to 378 K temperature range to determine their temperature-sensing properties.
The NPs containing Ho3+ and Er3+ ions revealed unusual behavior manifested by increased luminescence intensity with the temperature increase. This observation can be attributed to the specificity of the UC mechanism based on energy transfers from Ho3+ to Er3+ ions. Upon the research, we discovered that the NaYF4:Ho3+, Er3+@NaYF4 NPs have great potential as a temperature sensor based on the excitation and emission in the range of biological windows. This sample shows intense NIR luminescence from Ho3+ ions at 899 and 970 nm and Er3+ ions at 982 nm. The relative sensitivity determined for these peaks reached the maximum value of 1.80%/K at 378 K. This optical temperature sensor based on the NIR UC emission of the system containing Ho3+ and Er3+ ions has been reported for the first time. The possibility of excitation within the second biological window and detecting temperature changes in emission intensity around the first biological window make our UCNPs promising candidates for biomedical applications. However, the obtained UCNPs presented high sensitivities not only in the NIR range, which generally makes them excellent candidates for temperature sensing applications not only limited to biological ones.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
References
Sharma, A. A. & Szaflarski, J. P. In vivo imaging of neuroinflammatory targets in treatment-resistant epilepsy. Curr. Neurol. Neurosci. Rep. 20, 5 (2020).
Liu, X. et al. Fast wide-field upconversion luminescence lifetime thermometry enabled by single-shot compressed ultrahigh-speed imaging. Nat. Commun. 12, 1–9 (2021).
Shen, Y. et al. Reliable and Remote Monitoring of Absolute Temperature during Liver Inflammation via Luminescence-Lifetime-Based Nanothermometry. Adv. Mater. 34, 2107764 (2022).
Mohanty, S. et al. Dual-mode vehicles with simultaneous thermometry and drug release properties based on hollow Y2O3:Er, Yb and Y2O2SO4:Er:Yb spheres. RSC Adv. 12, 33239–33250 (2022).
Meng, M. et al. NaYF4:Yb3+/Tm3+@NaYF4:Yb3+ upconversion nanoparticles for optical temperature monitoring and self-heating in photothermal therapy. ACS Appl. Nano Mater. 6, 759–771 (2023).
Kasymov, D. et al. Experimental investigation of the effect of heat flux on the fire behavior of engineered wood samples. Fire 3, 1–16 (2020).
Perný, M., Šály, V., Puzová, M., Váry, M. & Kujan, V. Thermographic diagnostics of a DC traction railway system, in 2018 19th International Scientific Conference on Electric Power Engineering (EPE), 1–5 (2018). https://doi.org/10.1109/EPE.2018.8396022
Zhu, W., Zhao, L., Jin, J. & Song, Y. Preparation of core-shell rare earth-doped upconversion nanomaterials and simultaneous detection of two pesticides in food. Foods 11, 1485 (2022).
Marin, R. et al. Luminescence thermometry using sprayed films of metal complexes. J. Mater. Chem. C 10, 1767–1775 (2022).
Liu, R. et al. Upconversion of Yb3+/Ho3+ co-doped bismuth oxyfluoride and fluoride microcrystals for high-performance ratiometric luminescence thermometry. J. Lumin. 258, 119791 (2023).
Wang, X. et al. Up-conversion luminescence, temperature sensing properties and laser-induced heating effect of Er3+/Yb3+ co-doped YNbO4 phosphors under 1550 nm excitation. Sci. Rep. 8, 5736 (2018).
Ximendes, E., Marin, R., Carlos, L. D. & Jaque, D. Less is more: Dimensionality reduction as a general strategy for more precise luminescence thermometry. Light Sci. Appl. 11, 237 (2022).
Li, L. et al. Boltzmann-distribution-dominated persistent luminescence ratiometric thermometry in NaYF4:Pr3+. Opt. Lett. 47, 1701 (2022).
Spencer, J. Q. & Sanderson, D. C. W. Mapping thermal exposure by luminescence thermometry. Radiat. Meas. 23, 465–468 (1994).
Samal, S. K., Pushpendra, Y. J. & Naidu, B. S. Upconversion properties of Er, Yb co-doped KBi(MoO4)2 nanomaterials for optical thermometry. Ceram. Int. 49, 20051–20060 (2023).
Wang, S., Westcott, S. & Chen, W. Nanoparticle luminescence thermometry. J. Phys. Chem. B 106, 11203–11209 (2002).
Bednarkiewicz, A., Marciniak, L., Carlos, L. D. & Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 12, 14405–14421 (2020).
Lozano-Gorrín, A. D. et al. Lanthanide-doped Y3Ga5O12 garnets for nanoheating and nanothermometry in the first biological window. Opt. Mater. 84, 46–51 (2018).
Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 104, 139–174 (2004).
Carnall, W. T., Crosswhite, H. & Crosswhite, H. M. Energy level structure and transition probabilities in the spectra of the trivalent lanthanides in LaF3. Energy https://doi.org/10.2172/6417825 (1978).
Bünzli, J.-C.G. & Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 34, 1048–1077 (2005).
Quintanilla, M. et al. Cubic versus hexagonal – phase, size and morphology effects on the photoluminescence quantum yield of NaGdF4:Er3+/Yb3+ upconverting nanoparticles. Nanoscale 14, 1492–1504 (2022).
Pu, L. et al. Eu3+ -activated single-band ratiometric nanothermometry by lattice negative thermal expansion. Laser Photon. Rev. https://doi.org/10.1002/lpor.202200884 (2023).
Zheng, W. et al. Lanthanide-doped upconversion nano-bioprobes: Electronic structures, optical properties, and biodetection. Chem. Soc. Rev. 44, 1379–1415 (2015).
Pandey, A. Rare earth doped materials for temperature sensors. Spectrosc. Tech. Secur. Forensic Environ. Appl. 279–292 (2014).
Krishnaraj, C., Rijckaert, H., Jena, H. S., Van Der Voort, P. & Kaczmarek, A. M. Upconverting Er3+–Yb3+ inorganic/covalent organic framework core-shell nanoplatforms for simultaneous catalysis and nanothermometry. ACS Appl. Mater. Interfaces 13, 47010–47018 (2021).
Du, P., Deng, A. M., Luo, L. & Yu, J. S. Simultaneous phase and size manipulation in NaYF4:Er3+/Yb3+ upconverting nanoparticles for a non-invasion optical thermometer. New J. Chem. 41, 13855–13861 (2017).
Wang, J. et al. A novel high-sensitive upconversion thermometry strategy: Utilizing synergistic effect of dual-wavelength lasers excitation to manipulate electron thermal distribution. Sens. Actuators B Chem. 278, 165–171 (2019).
Antoniak, M. A. et al. Combined temperature and pressure sensing using luminescent NaBiF4:Yb, Er Nanoparticles. ACS Appl. Nano Mater. 3, 4209–4217 (2020).
Feng, Z., Lin, L., Wang, Z. & Zheng, Z. Highly efficient and wide range low temperature sensing of upconversion luminescence of NaYF4:Er3+ nanoparticles: Effects of concentration of active or sensitive ions, excitation power and particle size on temperature sensing sensitivity. Opt. Commun. 491, 126942 (2021).
Ryszczyńska, S., Trejgis, K., Marciniak, Ł & Grzyb, T. Upconverting SrF2:Er3+ nanoparticles for optical temperature sensors. ACS Appl. Nano Mater. 4, 10438–10448 (2021).
Shao, L., Zhao, Y., Wang, X. & Li, Y. Up-conversion luminescence and temperature sensing properties of Ho3+/Gd3+ co-doped YbNbO4 phosphors. Mater. Res. Bull. 144, 111495 (2021).
Xu, W. et al. Optical temperature sensing through the upconversion luminescence from Ho3+/Yb3+ codoped CaWO4. Sens. Actuators B Chem. 188, 1096–1100 (2013).
Liu, Q. et al. Effect of the Yb3+ concentration in up-conversion and electrical properties of Ho3+/Yb3+ Co-doped (0.94Na0.5Bi0.5TiO3–0.06BaTiO3) ceramics. J. Electron. Mater. 45, 3473–3478 (2016).
Zhou, H. et al. Optical temperature sensing properties of Tm3+/Yb3+ co-doped LuAG polycrystalline phosphor based on up-conversion luminescence. J. Lumin. 229, 117656 (2021).
Suo, H. et al. All-in-one thermometer-heater up-converting platform YF3:Yb3+, Tm3+ operating in the first biological window. J. Mater. Chem. C 5, 1501–1507 (2017).
Gandhi, Y. et al. Influence of aluminum ions on fluorescent spectra and upconversion in codoped CaF2-Al2O3-P2O5-SiO2: Ho3+ and Er3+ glass system. J. Appl. Phys. https://doi.org/10.1063/1.3464257 (2010).
Cheng, X. et al. Design for brighter photon upconversion emissions via energy level overlap of lanthanide ions. ACS Nano 12, 10992–10999 (2018).
Suyver, J. F. et al. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+. J. Lumin. 117, 1–12 (2006).
Haye, L. et al. Enhancing near infrared II emission of gold nanoclusters via encapsulation in small polymer nanoparticles. Adv. Opt. Mater. 11, 2201474 (2022).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 32, 751–767 (1976).
Sevic, D. et al. Luminescence thermometry based on Y2O2S:Er, Yb nanophosphor. Opt. Quantum Electron. 54, 523 (2022).
Avram, D. & Tiseanu, C. Thermometry properties of Er, Yb–Gd2O2S microparticles: Dependence on the excitation mode (cw versus pulsed excitation) and excitation wavelength (980 nm versus 1500 nm). Methods Appl. Fluoresc. 6, 025004 (2018).
Solanki, P. S., Balabhadra, S., Reid, M. F., Golovko, V. B. & Wells, J. P. R. Upconversion thermometry using Yb3+/Er3+Co-doped KY3F10 nanoparticles. ACS Appl. Nano Mater. 4, 5696–5706 (2021).
Pollnau, M., Gamelin, D., Lüthi, S., Güdel, H. & Hehlen, M. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 61, 3337–3346 (2000).
Lakowicz, J. R. Principles of Fluorescence Spectroscopy (Springer, 2006).
Peng, Y. et al. Intense single-band red upconversion emission in BiOCl:Er3+ layered semiconductor via co-doping Ho3+. J. Rare Earths 38, 577–583 (2020).
Chan, E. M. et al. Combinatorial discovery of lanthanide-doped nanocrystals with spectrally pure upconverted emission. Nano Lett. 12, 3839–3845 (2012).
Liu, O. K., Chen, Y. H. & Beitz, J. V. Photon avalanche up-conversion in Ho3+ doped fluoride glasses. J. Lumin. 81, 7–12 (1999).
Karmakar, B. IRRS, UV-Vis-NIR absorption and photoluminescence upconversion in Ho3+-doped oxyfluorophosphate glasses. J. Solid State Chem. 178, 2663–2672 (2005).
Dwivedi, Y. & Rai, S. B. Photon avalanche upconversion emission in Ho: Gd2O3 nanophosphor. Int. J. Nanosci. 10, 925–928 (2011).
Acknowledgements
The National Science Centre, Poland, funded this research under grant no. 2016/22/E/ST5/00016 and project No. POWR.03.02.00-00-I032/16 under the European Social Fund – Operational Programme Knowledge Education Development, Axis III Higher Education for Economy and Development, Action 3.2 Ph.D. Programme.
Author information
Authors and Affiliations
Contributions
S.R. Conceptualization, Methodology, Investigation, Writing—Original Draft, Writing—review and editing, Visualization. I.R.M. Methodology, Investigation, Writing—Original Draft. T.G.: Conceptualization, Investigation, Writing—review and editing, Supervision, Funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryszczyńska, S., Martín, I.R. & Grzyb, T. Near-infrared optical nanothermometry via upconversion of Ho3+-sensitized nanoparticles. Sci Rep 13, 14819 (2023). https://doi.org/10.1038/s41598-023-42034-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-42034-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.