Abstract
The conversion of NIR light into visible light has been studied in Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphor for the first time. The crystallinity and particles size of the phosphor increase through Bi3+ doping. The absorption characteristics of Ho3+, Yb3+ and Bi3+ ions are identified by the UV–vis-NIR measurements. The Ho3+ doped phosphor produces intense green upconversion (UC) emission under 980 nm excitations. The emission intensity ~ excitation power density plots show contribution of two photons for the UC emissions. The UC intensity of green emission is weak in the Ho3+ doped phosphor, which enhances upto 128 and 228 times through co-doping of Yb3+ and Yb3+/Bi3+ ions, respectively. The relative and absolute temperature sensing sensitivities of Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 phosphor are calculated to be 13.6 × 10−4 and 14.3 × 10−4 K−1, respectively. The variation in concentration of Bi3+ ion and power density produces excellent color tunability from green to red via yellow regions. The CCT also varies with concentration of Bi3+ ion and power density from cool to warm light. The color purity of phosphor is achieved to 98.6% through Bi3+ doping. Therefore, the Ho3+/Yb3+/Bi3+:ZnGa2O4 phosphors can be suitable for UC-based color tunable devices, green light emitting diodes and temperature sensing.
Similar content being viewed by others
Introduction
The zinc gallate (ZnGa2O4) based phosphors are very promising photoluminescent materials due to their unique low phonon energy. This permits large photoluminescence intensity of the lanthanide ions for various exciting applications, such as display devices, field emission display devices (FEDs), temperature sensing, color tunable devices, induced optical heating, bio-imaging, etc1,2,3,4,5. The ZnGa2O4 is a self-activated photoluminescent material for solid state lighting6. The lanthanide-based ZnGa2O4 material gives large photoluminescence of the narrow band emissions4,5. These emissions arise due to ladder-like energy levels present in the lanthanide ions7,8,9,10,11. In various lanthanide ions, the combination of Ho3+/Yb3+ ions has been found interesting to investigate the upconversion (UC) properties in different host materials12,13,14,15. It has been found that this combination yields strong UC emission intensity because of energy transfer between Ho3+ and Yb3+ ions. In this case, the Yb3+ ion acts as sensitizer. The emission intensity of phosphor materials could also be enhanced by incorporating trace amount of some dopant ions, for example Li+, Mg2+, Zn2+, Ca2+, etc15,16,17.
The photoluminescence properties of Ho3+/Yb3+ activated phosphors were improved considerably in recent years by adding different dopant ions, which play the role of surface modifiers and sensitizers in the host materials16,17. The dopant ions, i.e. Li+, Mg2+, Zn2+, Bi3+ and Cr3+ act as surface modifiers15,16,17,18,19. These ions have modified local crystal structure around the acceptor ions for better emission intensity in the materials. Out of these, the Bi3+ ion has been used as surface modifier to improve the UC intensity of Er3+/Yb3+ activated La2O3 material7. Alternatively, the Bi3+ ion has also been selected as sensitizer in the downshifting (DS) process in which it transfers its energy to the Dy3+ and Tb3+ ions in the YPO4 and Y2O3 phosphor materials, respectively. This improves the emission intensity of the phosphor materials20,21. Thus, the Bi3+ ion is a promising material to increase the photoluminescence intensity of phosphor samples for the UC and DS processes7,21. The increment of UC intensity in the Ho3+/Yb3+ activated phosphor samples were observed by our group in the presence of Li+ and Mg2+ ions15,16. However, Kumar et al. have investigated the improvement in UC emissions of the Ho3+/Yb3+:Gd2O3 phosphor in the presence of Ca2+/Zn2+ ions17. Moreover, Cheng et al. have discussed UC process of the Er3+/Yb3+:ZnGa2O4 phosphor in the presence of Cr3+ ion18. The increase in emission intensity of the phosphor has been also observed through doping of Bi3+ ion19. The UC emission intensity of Ho3+/Yb3+:ZnGa2O4 material was enhanced significantly through doping of Li+ ion22. However, the emissive properties of Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphor remains unexplored to our knowledge.
The UC emissions of phosphor materials can further be used to investigate their application in temperature sensing. The temperature sensing process is generally related to the fluorescence intensity ratio (FIR) of two close lying thermally coupled levels (i.e. TCLs)3,4,7,8. The range of energy gap is usually 100–2000 cm−1 for TCLs and it is quite different for different lanthanide ions4,13. These levels are affected by a small variation in external temperatures of the phosphor, which influences the intensity of emission bands originated from the TCLs3,8,13. The rise in temperature would increase the lattice vibrations and this leads to shift of some excited ions from a lower level to the upper level of TCLs. The temperature sensing properties have been investigated by many groups of workers in various sets of the lanthanide co-doped phosphor materials3,4,7,8,13. It was noticed that a change in intensity of the emission bands occurs due to increase in temperature of the phosphors. Chai et al13 have studied the UC-based temperature sensing in Ho3+/Yb3+:ZnWO4 phosphor. The intensity of green bands decreases regularly with the increase in temperature of the phosphor. However, the optical thermometry has also been reported by Kumar et al. in Ho3+/Yb3+:Gd2O3 phosphor through incorporation of Ca2+/Zn2+ ions using TCLs of Ho3+ ion17. Moreover, the temperature sensing properties was also reported by Lojpur et al. in Ho3+/Yb3+:Y2O3 phosphor23. As has been mentioned earlier, the UC intensity of Ho3+/Yb3+/Bi3+ activated ZnGa2O4 material has been not investigated. In addition to this, the temperature sensing properties is also not studied in the Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphor to our knowledge.
Color tunability is a very fascinating property of the lanthanide ions. It occurs due to change in number of the ions in different higher energy states. The color tunability was observed not only in the downshifting (DS) but also in the UC-based phosphor materials2,4,13,24,25. The variations in concentration and excitation wavelength of the Tb3+ based DS phosphor showed color tunability9. The change in concentrations of the lanthanide ions in the UC-based phosphors also showed color tunability5. The color tunability features of Ho3+/Yb3+ activated UC phosphor samples were reported by exciting them at different power densities of 980 nm13,25. Our group has also reported the influence of concentrations as well as power densities on the UC intensity and obtained distinct color tunability features in the Er3+/Yb3+ based UC phosphor4. However, it would also be interesting to understand the influence of dopant concentration as well as power density on the color tunability of Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphor.
In this paper, the Ho3+; Ho3+/Yb3+ and Ho3+/Yb3+/Bi3+ doped and co-doped ZnGa2O4 phosphor materials have been prepared by using solid-state reaction method. The X-ray diffraction (XRD), scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) measurements have been used for identifying the phase formation, crystallinity, crystallite size, particles shape and size, and elemental traces present in the ZnGa2O4 materials. The Fourier transform infrared (FTIR) measurements were performed to confirm phonon energy of the phosphor lattice. The UV–vis–NIR measurements reveal the absorption characteristics of Ho3+/Yb3+/Bi3+ ions in the samples. The Ho3+ doped phosphor sample gives relatively large green emission under 980 nm excitations. The emission intensity of phosphor increased significantly through doping of Yb3+ and Yb3+/Bi3+ ions. The UC intensity ~ power density and lifetime measurements have also been performed to know the mechanisms of UC process and decay behaviors of the phosphor materials, respectively. The temperature sensing sensitivity has also been observed in Ho3+/Yb3+/Bi3+ activated ZnGa2O4 sample. The color tunability, correlated color temperature (CCT) and color purity are also discussed with the concentration of Bi3+ ions and power density. The highly intense UC emissions in the Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphors can be found appropriate for the fabrication of UC based color tunable devices, green light emitting diodes (g-LEDs) and temperature sensors.
Results and discussion
Structural and morphological studies
XRD measurements
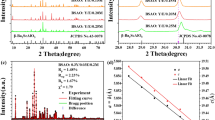
The XRD patterns of Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 phosphors examined in the 2θ region of 25–80° angles are given in Fig. 1. The sharp and intense XRD peaks are observed in both the cases, which show the crystalline nature of phosphor samples. The XRD patterns are well matched to JCPDS File number 38–12404,6. The phase of phosphor is confirmed to cubic with a space group of Fd \(\overline{3}\) m(227). The cell constants for cubic phase are identified as a = b = c = 8.334 Å and α = β = γ = 90°, respectively. However, some additional XRD peaks are observed due to the Ga5Yb3O12 compound (JCPDS File no. 73–1373). The crystallite size of Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped materials has been calculated from Debye–Scherrer formula21:
where D refers to crystallite size; λ (= 0.15406 nm) is the X-ray radiation wavelength; β shows FWHM (full width at half maximum) of the peaks in radian; θ is diffraction angle and k (= 0.90) is the shape factor. The crystallite size values are calculated to be 31.79 and 33.38 nm for the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ activated phosphor materials, respectively. Thus, the crystallinity of phosphor increases through Bi3+ doping. The increase in crystallinity can also be confirmed from the inset of Fig. 1 in which the FWHM of peak is reduced and shifted towards lower angle side through Bi3+ doping. This is attributed to larger ionic radius of Bi3+ ion (1.03 Å) compared to Zn2+ ion (0.74 Å). This indicates that the Bi3+ doping does not affect the phase of sample; however, it increases crystallinity of the Ho3+/Yb3+ co-doped sample.
The XRD patterns of the Ho3+/Yb3+ co-doped ZnGa2O4 phosphor materials with and without the Bi3+ ion and the inset of Fig. 1 shows a variation of FWHM and a shift in XRD peak for 63.06° angle.
The dislocation density of the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 materials has also been calculated by using the following relation26:
where δ is the dislocation density, which reduces with the increase of crystallite size of phosphor. The dislocation density is found to be 9.9 × 1014 and 8.9 × 1014 m−2 with respect to the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped phosphor materials, respectively. This confirms that the dislocation density of phosphor decreases through Bi3+ doping. This also indicates an enhancement of local crystal structure around the lanthanide ions in phosphor. The microstrain (e) is also evaluated in the two phosphor materials by using the following relation27:
where the terms show the usual meaning. The values of microstrain are obtained as 11.4 × 10−2 and 10.8 × 10−2 for the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped phosphor materials, respectively. It shows that the microstrain of phosphor reduces via doping of Bi3+ ion. Therefore, the XRD analyses elaborate that not only growth in crystallinity but also a decrease in dislocation density and microstain would be supportive for getting large UC intensity from the phosphor materials.
SEM and EDS analyses
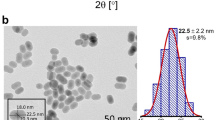
Figure 2 represents the SEM images of the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 phosphor materials. The particles of phosphors are found in random manners with the agglomerated features. The particles shape of phosphor is changed to the flower-like structure through Bi3+ doping. However, the particles size of phosphor material is observed to increase (see Fig. 2b). The change in particles shape and size of different host materials has been discussed by the other researchers in the presence of different surfactants and dopant ions28,29. The formation of larger sized particles was also observed by our group and Wu et al. in the Er3+/Yb3+:La2O3 and Er3+:Y2O2S phosphor materials, respectively through Bi3+ doping7,30. In our case, the average value of particles size is obtained as 0.70 µm for the Ho3+/Yb3+ co-doped phosphor and it increases to 0.82 µm through Bi3+ doping. Thus, the particles shape and size of phosphor are modified through Bi3+ doping.
Figure 3a,b depict the EDS spectra of Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 phosphor materials. The spectra reveal that the phosphor materials contain Bi, Ga, Ho, O, Yb and Zn elements. Figure 3c,h show the EDS mappings of the Zn, Ga, Ho, Yb, Bi and O constituents in the Ho3+/Yb3+/5Bi3+ co-doped material generated by using INCA software. These figures suggest that all the constituents are distributed almost uniformly in the phosphor sample. The distribution of these elements in the phosphor sample would be more suitable for getting larger UC emission intensity.
Optical measurements
FTIR studies
The molecular vibrational groups existing in the phosphor materials have been studied by the FTIR measurements. The FTIR spectra of Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped ZnGa2O4 materials have been monitored in 400–4000 cm−1 range as revealed in Fig. 4. The vibrational frequencies are observed at 413 and 569 cm−1 corresponding to the stretching modes of the ZnO and GaO groups, respectively4,6. The position of different bands remains unchanged through Bi3+ doping; however, the intensity of these bands varies accordingly. Since the phosphor sample was prepared at higher temperature (at 1200 °C) the impurity peaks, such as OH− and CO32− groups, etc. do not appear in the spectra4. The figure also indicates that the phonon frequency of ZnGa2O4 host is small and hence, the photoluminescence intensity of radiative transitions would be large in the phosphor materials.
UV–vis–NIR studies
Figure 5 illustrates the UV–vis-NIR absorption spectra of the Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped phosphor materials monitored in 200–1100 nm range using diffuse reflectance mode. The band at 240 nm has been assigned to the charge transfer state (CTS) of O2− → Ga3+ corresponding to ZnGa2O4 host4. Alongwith this, the spectra have different absorption peaks positioned at 366, 419, 454, 487, 540 and 639 nm because of various transitions of the Ho3+ ions, which are attributed through absorption from ground state (5I8) to higher excited states, such as 3H6, 5G6, 3K8, 5F3, (5F4/5S2) and 5F5, respectively16. After doping the Bi3+ ion in phosphor, a broad absorption band is also observed in the 230–405 nm region and it is the overlapped profiles of CTS of O2− → Ga3+ ions and the absorption from 1S0 level to 1P1 (at 283 nm) and 3P1 (at 401 nm) levels of the Bi3+ ions7,21.
An intense broad absorption band has also been found at 971 nm because of 2F7/2 → 2F5/2 transition of the Yb3+ ion16. The absorption cross-section of NIR region is improved considerably due to increase in crystallinity of the phosphor through Bi3+ doping, which is favorable for the large excitation and radiative transitions. Since the absorption band of Yb3+ ion is very broad it can absorb large number of incident photons, which would generate the large UC intensity of Ho3+ ion.
Optical band gap analysis
Optical band gap of Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 materials can be estimated by using Wood–Tauc formula31:
where Eg refers to the band gap energy; hυ is the energy of incident photons; α is absorption coefficient and B is band tailoring constant. The ‘n’ value was chosen as (1/2) for the direct allowed transitions. The plotted graphs of hυ ~ (αhυ)2 for the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ activated phosphors are given in Fig. 6. The values of band gap energy (Eg) are obtained as 4.80 and 4.70 eV for the Ho3+/Yb3+/0Bi3+ and Ho3+/Yb3+/5Bi3+ activated phosphor materials, respectively.
This means that the band gap of Ho3+/Yb3+ co-doped ZnGa2O4 material decreases via Bi3+ doping21. It has been mentioned above that not only the crystallite size but the particles size of phosphor is also improved via Bi3+ doping. An increase in particles size will also reduce the gap between the valence and the conduction bands in ZnGa2O4 lattice. If the band gap of phosphor reduces; the large numbers of the excited ions will be transferred to the higher energy states, which would generate better UC intensity for the ZnGa2O4 materials.
Upconversion studies
The upconversion emission spectra of the Ho3+/0Yb3+ and Ho3+/3Yb3+ doped and co-doped ZnGa2O4 materials monitored in 450–800 nm range under 980 nm excitations at 31.84 W/cm2 are revealed in Fig. 7. In the figure, the emission spectra possess several emission peaks in the blue, green, red and NIR regions. They are centered at 486, (537, 538), 547, 664 and 755 nm and attributed to 5F3 → 5I8, 5F4 → 5I8, 5S2 → 5I8, 5F5 → 5I8 and 5S2 → 5I7 transitions of the Ho3+ ion, respectively12,32,33,34,35. The Ho3+ doped phosphor produces weak transitions in the green and the red regions22. The UC intensity of the green emission is many times higher than the red emission. However, the blue as well as NIR emissions are not found due to lack of excitation. Dey et al33 have also reported similar type of emissions in the green, red and NIR regions for Ho3+ doped CaMoO4 phosphor. The inset of Fig. 7 shows an enlarged spectrum of Ho3+ doped ZnGa2O4 phosphor for the green region.
On the other hand, these emissions could appear through doping of Yb3+ ion alongwith Ho3+ ion in the ZnGa2O4 host. The Ho3+ and Yb3+ co-doped phosphor leads to distinct appearance of not only the green and red emissions but also the blue and NIR emissions. The UC intensity of Ho3+ doped phosphor is improved many times in presence of Yb3+ ion. This attributes to the energy transfer (ET) from Yb3+ to Ho3+ ions12,13,14,15,16,17,33,35. The emission intensity of green color is several times stronger than the blue, red and NIR emissions. Chen et al. have also found the similar trend of UC intensity for these emissions in the SrF2:Gd3+/Yb3+/Er3+ nanocrystals36. Further, the emission intensity of red band is larger than the blue and NIR bands. Hence, the UC intensity of Ho3+ doped phosphor is increased by 128, 67 and 21 times in the presence of Yb3+ ion for the green, red and NIR emissions, respectively. The Yb3+ ion, thereby acts as sensitizer for the Ho3+ doped phosphor.
Power density dependent studies
The UC intensity of the Ho3+/Yb3+ co-doped ZnGa2O4 phosphor material has been monitored at various excitation power densities of 980 nm radiation. The UC emission is a nonlinear process and it is directly related to (nth) power of incident radiation8,37 e.g.
where n indicates the number of photons participating in the UC emission, Iup is the upconversion intensity and P is excitation power density in W/cm2. The dual logarithmic plots between the emission intensity ~ excitation power density for the green, red and NIR emissions of the Ho3+/Yb3+ co-doped material are given in Fig. 8. The emission intensity varies linearly with excitation power density upto certain limit and saturates due to involvement of non-radiative channels at higher excitation power density. The slope values (n) have been evaluated by linear fittings of dual logarithmic plots. These values are found to be 2.12, 2.35 and 1.96 for the green, red, and NIR emissions, respectively. From this, it has been noted that the 5F4 (green), 5F5 (red) and 5S2 (NIR) levels are populated by the absorption of two photons15. The deviation in an integer value occurs because of non-radiative processes engaged for populating these levels. The mechanisms involved for these transitions can be discussed by using energy level diagrams of the Ho3+ and Yb3+ ions.
Figure 9 represents the distinct energy level diagrams of the Ho3+ and Yb3+ ions. When the Ho3+ doped ZnGa2O4 sample is excited with 980 nm photons it absorbs this radiation weakly either through phonon assisted excitation or via collision or both; because the Ho3+ ion has no resonant energy level with respect to 980 nm radiation. Due to this, a small number of the ions are shifted from ground state (5I8) to higher state (5I6) via ground state absorption (GSA) process. The Ho3+ ion present in 5I6 level reabsorbs 980 nm radiations and thereby populated the (5F4/5S2) excited states via excited state absorption (ESA) process. The excited ions in these states produce weak radiation in the green region22,33. Some ions are relaxed non-radiatively to populate the 5F5 state and due to this, a very weak red emission takes place. However, the emissions in the blue and NIR regions are not clearly identified due to lack of excitation. These emissions are clearly detected in the presence of Yb3+ ion (see Fig. 7).
The Yb3+ sensitizer ions transfer its excitation energy to Ho3+ ions via cooperative energy transfer (CET) and energy transfer upconversion (ETU) processes as can be seen from Fig. 9. When the Yb3+ and Ho3+ ions are added together in the ZnGa2O4 material, it gives strong emission because of ET from Yb3+ to Ho3+ ions. Actually, the excited level of Yb3+ ion is well matched with 980 nm radiation4,11. Therefore, on exciting the Ho3+/Yb3+ co-doped ZnGa2O4 sample by 980 nm diode laser the Yb3+ ions are promoted to its excited state (2F5/2) from the ground state (2F7/2). The Yb3+ ions thus transfer their excitation energy to the Ho3+ ions via ETU/CET processes, which promote them to different excited states, i.e. 5I6, 3K8 and 3K7 states. Thus, the population of Ho3+ ions in 5I6 state is increased enormously through GSA/ETU processes. The ions in the 5I6 state reabsorb 980 nm radiations and they jumped to (5F4/5S2) excited states by ESA/ETU processes. These excited states are further populated by the non-radiative transitions of ions from 3K8 state because 3K8 state is populated by ETU and CET processes. In CET process, two Yb3+ ions in the excited state combine together and transfer its energy to Ho3+ ions simultaneously12,13,14,15,16,17. Thus, the excited (5F4/5S2) states are populated with huge number of the Ho3+ ions and they return to the ground state by emitting strong green emission peaks at 537 and 547 nm. The weak NIR emission also occurs at 755 nm from 5S2 state to 5I7 state transition. Some of the ions present in (5F4/5S2) states are relaxed non-radiatively to 5F5 state. Due to this, a relatively weak red emission has been observed at 664 nm because of 5F5 → 5I8 transition. Finally, the 3K7 level is populated through ETU process and these ions relaxed non-radiatively to 5F3 level. The ions present in 5F3 level produce weak blue emission at 486 nm. Thus, the blue, green, red and NIR emissions are detected distinctly due to absorption of three/two NIR photons in different excited states32,33,34,35.
Effect of Bi3+ doping
As have been mentioned earlier, the Bi3+ ion is a very effective dopant, which has often been used as sensitizer as well as the surface modifier7,19. In order to understand the impact of Bi3+ ion on the UC intensity of Ho3+/Yb3+ co-doped samples, we have prepared the phosphors with various concentrations of Bi3+ ion (i.e. 3, 5, 7 and 10 mol%) and monitored their UC emission intensity in 450–800 nm range under 980 nm excitations at 31.84 W/cm2 power density. It has been noticed that the UC intensity of the samples enhances appreciably. Figure 10a depicts the UC emission spectra of Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped phosphors monitored under 980 nm excitations. The UC emission peaks observed via Bi3+ doping is similar to those observed in Ho3+/Yb3+ co-doped ZnGa2O4 material. However, the UC intensity of emission peaks is improved by several times. Firstly, the emission intensity is observed to enhance for 3 and 5 mol% concentrations of Bi3+ ion and it is larger for 5 mol% concentrations. The further increase in the concentrations of Bi3+ ion tends to a decrement in the UC intensity (i.e. for 7 and 10 mol%) due to concentration quenching. In this process, the excitation energy is lost in terms of multi-polar interactions because of a shorter gap among the Ho3+/Yb3+ ions than their critical distance8,15,33. The similar observation has also been found by Li et al38 in the Sr2P2O7:Bi2+ material. The effect of Bi3+ doping was also studied by Xu et al39 in the Sm3+/Eu3+ coactivated Ca20Al26Mg3Si3O68 phosphor in which the concentration quenching also takes place at higher concentrations of Bi3+ ions. Our group has also studied the impact of Bi3+ ion on the UC intensity of Er3+/Yb3+ activated phosphors and observed concentration quenching after 5 mol% concentrations of Bi3+ ions7,19. Wang et al40 have also reported concentration quenching in the NaGdF4:2%Er3+ phosphor after 25 mol% concentrations of Ca2+ ions. In the present case, we have also observed concentration quenching above 5 mol% concentrations of Bi3+ ion. Therefore, the UC intensity of Ho3+/Yb3+ co-doped ZnGa2O4 phosphor material is optimum at 5 mol% concentration of Bi3+ ion.
(a) The UC spectra of the Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped phosphors monitored under 980 nm excitations at 31.84 W/cm2. (b) The comparison of UC emission intensities of Ho3+ doped, Ho3+/Yb3+ and Ho3+/Yb3+/5Bi3+ co-doped phosphors monitored under 980 nm excitations at 31.84 W/cm2.
Figure 10b shows the comparison of emission intensities between Ho3+ doped, Ho3+/Yb3+ and Ho3+/Yb3+/5Bi3+ co-doped phosphors under 980 nm excitations at 31.84 W/cm2. As discussed above, the UC intensity of Ho3+ doped phosphor is increased by 128, 67 and 21 times for the green, red and NIR emission bands through Yb3+ doping. This arises because of ET from Yb3+ to Ho3+ ions41. Moreover, we have again observed an improvement in the UC intensity by 228, 272 and 57.7 times for the green, red and NIR emission peaks, respectively through Yb3+/Bi3+ co-doping compared to the pure Ho3+ doped material. Similarly, the UC intensity of Yb3+/Er3+ activated Zn2SiO4 material was improved by several times via Bi3+ doping42. It means that the Bi3+ doping helps significantly to improve the UC intensity of different materials.
The large enhancement in UC intensity has been discussed by taking accounts of several important parameters through Bi3+ doping. The improvement in crystallite size from 31.79 to 33.38 nm, decrease in dislocation density from 9.9 × 1014 to 8.9 × 1014 m−2 and microstrain from 11.4 × 10−2 to 10.8 × 10−2 through Bi3+ doping has created a large crystalline structure around the Ho3+ and Yb3+ ions. The particles size of phosphor is relatively larger through Bi3+ doping (see Fig. 2b). The band gap energy of phosphor is also decreased through Bi3+ doping, which improves the rate of excitation of the ions from the ground state to the higher energy states because of smaller gap between valence and conduction bands (see Fig. 6b). This would be responsible for generating large UC intensity in the Ho3+/Yb3+ co-doped ZnGa2O4 phosphor. The intensity of vibrational bands of ZnGa2O4 phosphor also varies in the presence of Bi3+ ion. The absorption cross-section of NIR region is also improved considerably due to increase in crystallinity of the phosphor through Bi3+ doping, which is favorable for the large excitation and radiative transitions7,19. All these parameters together played an essential role for large enhancement in UC intensity of the phosphor through doping of Bi3+ ion.
Lifetime studies
The lifetime of 5F4 level in the Ho3+/Yb3+/xBi3+ (x = 0, 3, 5, 7 and 10 mol%) co-doped phosphors has been monitored by exciting them at 980 nm using 31.84 W/cm2 power density. The decay curve of Ho3+/Yb3+/xBi3+ co-doped materials are given in Fig. 11. These decay curves were fitted mono-exponentially according to the following relation4,16:
where I0 and I refer to the initial and final emission intensity for 0 and t times, respectively. The term (τ) refers to lifetime of the 5F4 level.
The values of lifetime have been calculated and found to be 160, 173, 196, 183 and 163 μs for the Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped materials, respectively. It is clear from Fig. 11 that the decay time of 5F4 level of the Ho3+ ion increases through Bi3+ doping16,19,21. This supports an increase in UC intensity generated from the phosphor sample. The lifetime value increases upto 5 mol% then found to decrease at higher concentrations (i.e. 7 and 10 mol%). The lifetime value is expected to increase because of improvement in local crystal structure around the Ho3+ and Yb3+ ions in the phosphor16.
Temperature sensing sensitivity in Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 phosphor
The intensity of emission bands strongly depends on the temperature of phosphor sample, particularly the emission bands originating from two close lying thermally coupled levels (TCLs). The change in emission intensity can be realized by heating the sample externally. The intensity of emission bands changes on increasing temperature of the source40. If the TCLs of a lanthanide ion have a small separation it will be affected by a change in the temperature of sample3,7,13. It is well known that the green bands of Ho3+ ion arise due to the two TCLs, which are separated by 305 cm−1. It can sense a change of population between the two TCLs due to external heat given to the sample13,43. In our case, we have recorded the UC emission intensity of Ho3+/Yb3+/5Bi3+ co-doped ZnGa2O4 sample for two TCLs under 980 nm excitations at 12.73 W/cm2 in the range of 300–600 K temperature. Figure 12a depicts the temperature dependent UC emission intensity of two TCLs at 538 and 547 nm wavelengths in the region of 530–565 nm. On increasing the temperature from 300 to 600 K, a population shift of the excited ions takes place from lower level to the upper; however, the peak position of the bands remains unchanged. It is evident that the emission intensity of two TCLs decreases gradually with the rise in temperature44.
The emission peak arising from 5F4 level has two close lying Stark components at 537 and 538 nm wavelengths. We have selected the emission peak at 538 nm to estimate a change in the emission intensity with a temperature because it follows Boltzmann distribution law. In the beginning, the UC intensity of 547 nm is larger while it is smaller for 538 nm. Once the external temperature of phosphor is increased, the UC intensity of 547 nm decreases whereas it is found to increase for 538 nm. At 460 K, the emission intensities of both the peaks are almost the same. On increasing the temperature above 460 K, the intensity of 538 nm emission band is more than 547 nm. However, the overall emission intensities of two TCLs are decreased regularly with temperature. Similarly, Mahata et al45 have also used the TCLs of Ho3+ ion at 538 and 548 nm wavelengths and found that the UC intensity of two TCLs varies with the rise in temperature.
The UC emission intensities of TCLs at 538 and 547 nm wavelengths at various temperatures are given in Fig. 12b. The figure also clarifies that the emission intensity of 538 nm is initially smaller than 547 nm (during 300–440 K). The UC emission intensities of two peaks are almost equal at 460 K. However, the emission intensity of 538 nm peak is larger than 547 nm peak in the temperature range of 480–600 K. The change in emission intensity between 538 and 547 nm peaks can be taken to calculate the fluorescence intensity ratio (FIR), which is the basis for temperature sensitivity calculation13,43.
Figure 13a shows a plot between FIR (I538nm/I547nm) of TCLs and the temperature for the Ho3+/Yb3+/5Bi3+ co-doped phosphor under the excitation of 980 nm at 12.73 W/cm2. The FIR value rises from 0.95 to 1.08 with increasing temperature from 300 to 600 K, respectively. The nature of FIR (I538nm/I547nm) slope has been observed exponentially. The FIR values for (5F4) and (5S2) levels of Ho3+ ion follow Boltzmann distribution law and these values have been evaluated by using the following relation4,13,40:
where I1 and I2 stand for the emission intensity of two peaks arising from lower and upper TCLs, (k = 0.695 cm−1 K−1) is Boltzmann’s constant, △E is the energy difference between TCLs (i.e. 5F4 and 5S2 levels) and T refers to absolute temperature, respectively. Figure 13a also indicates that the values of FIR increase noticeably on increasing temperature of the ZnGa2O4 phosphor. Figure 13b reveals a plot of ln(FIR) versus (T−1) and it also follows the Boltzmann distribution law. This plot gives a slope value of 129 by linear fitting of the experimental data. The obtained value has been taken to calculate the temperature sensitivity in the both cases. Figure 13c,d show the plots between the relative (SR) and absolute (SA) temperature sensing sensitivities versus temperature for Ho3+/Yb3+/5Bi3+ co-doped sample, respectively. The temperature sensing sensitivities have been evaluated by taking the following relations13,43,46:
where the given terms show their usual meanings. The term ‘R’ is fluorescence intensity ratio (FIR) for the two peaks at 538 and 547 nm. We have calculated the relative and absolute sensitivities for different temperatures. The relative sensitivities are found to be 13.6 × 10−4 and 3.9 × 10−4 K−1 while the absolute sensitivities are 14.3 × 10−4 and 3.6 × 10−4 K−1 at 300 and 600 K temperatures, respectively. However, Mahata et al45 have reported the temperature sensitivity of 2.0 × 10−4 K−1 at 300 K in the Ho3+/Yb3+ activated BaTiO3 phosphor. Thus, the temperature sensing sensitivities of the prepared phosphor are better at lower temperature in the present case.
The values of temperature sensing sensitivity have also been observed by many groups of workers in various host materials17,45,47,48,49,50,51,52,53,54,55. We have also carried out a comparison of the temperature sensing sensitivity achieved in our case with the reported values by the other workers in Table 1. It has been concluded from this table that our temperature sensing sensitivity values are very close to the other reported values.
Color tunability, CCT and color purity analyses
The Commission International de l’Eclairage coordinates (CIE) have x and y parameters to determine the color tunability. The CIE diagram shows the hue and saturation in the two dimensional coordinates, which is also termed as the chromaticity diagram. The CIE diagrams were plotted for various concentrations of Bi3+ ion (i.e. x = 0, 3, 5, 7 and 10 mol%) in the Ho3+/Yb3+ co-doped materials under 980 nm excitations at 31.84 W/cm2 and also for the 12.73, 22.29 and 31.84 W/cm2 power densities of 980 nm in the Ho3+/Yb3+/10Bi3+ co-doped sample with the help of GoCIE 1931 software. Figure 14a reveals the CIE diagram of the Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped phosphor materials under 980 nm excitations at 31.84 W/cm2. The color emitted by phosphor materials is found to tune from green to red via yellow regions4. The CIE coordinates change from (0.27, 0.70) to (0.52, 0.41) on varying Bi3+ ion concentrations. This confirms that the color tunability in Ho3+/Yb3+ co-doped phosphor materials has been obtained through Bi3+ doping.
The CIE diagrams for (a) the Ho3+/Yb3+/xBi3+ co-doped ZnGa2O4 phosphor materials for various concentrations of Bi3+ ion (i.e. x = 0, 3, 5, 7 and 10 mol%) under 980 nm excitations at 31.84 W/cm2 and (b) for various power densities of 980 nm diode laser (i.e. 12.73, 22.29 and 31.84 W/cm2) in the Ho3+/Yb3+/10Bi3+ co-doped material using GoCIE 1931 software.
On the other hand, the emitted color of phosphor can also be changed on varying the power density of 980 nm source25. The CIE diagram of Ho3+/Yb3+/10Bi3+ co-doped material at various power densities of 980 nm i.e. 12.73, 22.29 and 31.84 W/cm2 is shown in Fig. 14b. At lower power density i.e. 12.73 W/cm2, the color of phosphor is green, which becomes yellow at 22.29 W/cm2. On further increase in the power density from 22.29 to 31.84 W/cm2, the emitted color is tuned from the yellow to red regions4,56. Therefore, the color coordinates of phosphors vary considerably with the rise in concentration and power density. The calculated CIE coordinates thus obtained in the two cases are also summarized in Table 2.
Basically, the CCT refers to correlated color temperature and it is used to show cool and warm nature of light. By using CIE coordinates (x, y) of the phosphors, we have also evaluated the CCT with the help of McCAMY’s formula. The CCT equation is written below4:
where n = (x − 0.3320)/(0.1858 − y) and (x, y) refer to the calculated values of CIE coordinates for the phosphors. The CCT values are obtained as 6395, 5831, 6113, 5976 and 2012 K for the Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped phosphor materials, respectively. The CCT value varies from cool light to the extra warm light. On varying the power density, the values of CCT of the Ho3+/Yb3+/10Bi3+ co-doped ZnGa2O4 material are found to be 5249, 4071 and 2012 K for the 12.73, 22.29 and 31.84 W/cm2, respectively (see Table 2). It shows that the CCT value also shifts from the natural light to the extra warm light with the power density57,58. Therefore, the Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphor is stable material and may be used for the applications of cool and warm LEDs.
Color purity is also one of the important parameter to realize the performance of a phosphor. The color purity has been calculated by the following relation59,60,61:
where (x, y), (xi, yi) and (xd, yd) are the CIE coordinates of phosphor, the standard light source and dominant wavelength, respectively. The values of color purity of the Ho3+/Yb3+/xBi3+ (i.e. x = 0, 3, 5, 7 and 10 mol%) co-doped phosphors are calculated to be 93.8, 98.6, 96.1, 95.8 and 79.1%, respectively (see Table 2). The color purity of phosphor is smaller for the Ho3+/Yb3+ doped sample. However, it is observed to increase through doping of Bi3+ ion. At higher concentrations of Bi3+ ion, the color purity is decreased61.
On the other hand, the color purity of Ho3+/Yb3+/10Bi3+ co-doped sample also decreases on varying the power density of 980 nm diode laser from 12.73 to 31.84 W/cm259. At low power density i.e. 12.73 W/cm2, the color purity of phosphor is found to be 94.9%. When the power density is changed from 22.29 to 31.84 W/cm2, the obtained values of color purity is decreased from 91.4 to 79.1%, respectively (see Table 2). It is clear from the above that color purity of a phosphor material is dependent on the Bi3+ ion concentrations and the power density. The lower value of the color purity refers to a shifting of the emitted light towards white region of the CIE diagram. Thus, the high color purity has been achieved to 98.6% in the phosphor through Bi3+ doping.
Conclusions
The Ho3+/Yb3+/xBi3+ co-doped ZnGa2O4 phosphor materials have been prepared by using solid state reaction method. The XRD analyses give an idea about the phase and crystalline nature of phosphors. The UV–vis–NIR absorption spectra show different bands of the Ho3+, Yb3+ and Bi3+ ions in the phosphors. The band gap of Ho3+/Yb3+ co-doped phosphor is reduced via doping of Bi3+ ion. The Ho3+ doped ZnGa2O4 phosphor emits intense green color under 980 nm excitations. The emission intensity of green band of the Ho3+ doped phosphor is increased upto 128 and 228 times through co-doping of Yb3+ and Yb3+/Bi3+ ions, respectively. This is attributed to energy transfer and improvement in local crystal structure of the phosphor. The relative and absolute temperature sensing sensitivities of Ho3+/Yb3+/5Bi3+ co-doped phosphor are found as 13.6 × 10−4 and 14.3 × 10−4 K−1 at 300 K, respectively. The CIE diagrams of phosphors show excellent color tunability with high color purity of 98.6% through doping of Bi3+ ion. The CCT value of phosphors shifts from the cool light to the extra warm light. Therefore, the Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphors can be useful in UC based color tunable devices, green LEDs and as temperature sensors.
Experimental method
Synthesis
The Ho3+, Yb3+ and Bi3+ doped and co-doped ZnGa2O4 phosphors have been prepared by solid state reaction method4. The starting materials used were Ho2O3 (99.99%), Yb2O3 (99.99%), ZnO (99.99%), Ga2O3 (99.99%) and Bi2O3 (99%). The Ho3+/Yb3+/xBi3+ co-doped phosphors have been prepared with the fixed concentrations of Ho3+ and Yb3+ ions and these are kept at 1 and 3 mol%, respectively. The concentrations of Bi3+ ion were varied as x = 3, 5, 7 and 10 mol%. The starting materials were weighed carefully and mixed completely in the agate mortar by taking acetone as a mixing agent. The homogeneously mixed powder was placed in an alumina crucible and then heated within the closed furnace at 1200 °C for 5 h. The heating temperature was constant for all the materials. The obtained materials are crushed properly in the agate mortar to form fine powders. The obtained powders are used for the structural and optical studies.
Instrumentation
The XRD measurements were carried out to study the crystalline nature and phase purity of the phosphor materials by using CuKα radiation (λ = 0.15406 nm) based Rigaku diffractometer system. The surface morphology of phosphor was studied by SEM (Zeiss, Evo18 Research). The presence of different constituents in the phosphor samples was documented by EDS technique. The EDS mapping images were generated by using INCA software attached with INCAx-act Oxford Instruments (51-ADD0048). The UV–vis–NIR absorption spectra were studied in diffuse reflectance mode with the help of Perkin Elmer Lambda-750 (Ultraviolet–visible-Near infrared spectrometer) unit in the 200–1100 nm region. The FTIR spectra were monitored by using a Perkin Elmer IR spectrometer (I Frontier unit) in 400–4000 cm−1 range. The upconversion emission spectra were monitored with the help of 980 nm and also iHR320 Horiba Jobin Yvon spectrometer attached with PMT. The decay curves for 5F4 level of the Ho3+ ion were monitored by chopping continuous beam of 980 nm radiations with the help of a mechanical chopper and 150 MHz digital oscilloscope of Hameg instruments using Model No. HM1507. Finally, the phosphor materials were heated outside with the digital thermo-couple arrangements for analyzing the temperature sensing capability. The CIE diagrams of the phosphor samples were drawn with the help of GoCIE 1931 software.
References
Lee, J. H. et al. Characteristics of nano-sized ZnGa2O4 phosphor prepared by solution combustion method and solid-state reaction method. J. Eur. Cer. Soc. 27, 965–968 (2007).
Yang, W. et al. Multi-wavelength tailoring of ZnGa2O4 nanosheet phosphor via defect engineering. Nanoscale 10, 19039–19045 (2018).
Xu, W., Hu, Y., Zheng, L., Zhang, Z. & Cao, W. Improved green upconversion emissions from CaWO4: Er3+-Yb3+ by Cr3+ codoping for optical thermometry. J. Lumin. 215(116617), 1–10 (2019).
Monika, Yadav, R. S., Bahadur, A. & Rai, S. B. Concentration and pump power-mediated color tunability, optical heating and temperature sensing via TCLs of red emission in an Er3+/Yb3+/Li+ co-doped ZnGa2O4 phosphor. RSC Adv. 9, 40092–40108 (2019).
Hussen, M. K. & Dejene, F. B. Effect of Cr3+ doping on structural and optical property of ZnGa2O4 synthesized by sol gel method. Optik 181, 514–523 (2019).
Garcia, C. R. et al. Controlling the white phosphorescence ZnGa2O4 phosphors by surface defects. Ceram. Int. 45, 4972–4979 (2019).
Yadav, R. S., Kumar, D., Singh, A. K., Rai, E. & Rai, S. B. Effect of Bi3+ ion on upconversion-based induced optical heating and temperature sensing characteristics in the Er3+/Yb3+ co-doped La2O3 nano-phosphor. RSC Adv. 8, 34699–34711 (2018).
Yadav, R. S., Dhoble, S. J. & Rai, S. B. Enhanced photoluminescence in Tm3+, Yb3+, Mg2+ tri-doped ZnWO4 phosphor: Three photon upconversion, laser induced optical heating and temperature sensing. Sens. Actuat. B: Chem. 273, 1425–1434 (2018).
Yadav, R. S. & Rai, S. B. Concentration and wavelength dependent frequency downshifting photoluminescence from a Tb3+ doped yttria nano-phosphor: A photochromic phosphor. J. Phys. Chem. Solids 114, 179–186 (2018).
Yu, D. et al. Understanding and tuning blue-to-near-infrared photon cutting by the Tm3+/Yb3+ couple. Light Sci. Appl. 9, 107 (2020).
Qin, W. P. et al. Multi-ion cooperative processes in Yb3+ clusters. Light Sci. Appl. 3(e193), 1–6 (2014).
Li, X., Zhu, J., Man, Z., Ao, Y. & Chen, H. Investigation on the structure and upconversion fluorescence of Yb3+/Ho3+ co-doped fluorapatite crystals for potential biomedical applications. Sci. Rep. 4, 4446 (2014).
Chai, X., Li, J., Wang, X., Li, Y. & Yao, X. Upconversion luminescence and temperature sensing properties of Ho3+/Yb3+codoped ZnWO4 phosphors based on fluorescence intensity ratios. RSC Adv. 7, 40046–40052 (2017).
Liu, S. et al. Investigation on the upconversion luminescence in Ho3+/Yb3+ co-doped Ba3Sc4O9 phosphor. Mater. Res. Bull. 98, 187–193 (2018).
Yadav, R. V. et al. Effect of Li+ on frequency upconversion and intrinsic optical bistability of Ho3+/Yb3+ co-doped gadolinium tungstate phosphor. J. Phys. Chem. Solids 119, 138–146 (2018).
Maurya, A., Yadav, R. S., Yadav, R. V., Bahadur, A. & Rai, S. B. Enhanced green upconversion photoluminescence from Ho3+/Yb3+ co-doped CaZrO3 phosphor via Mg2+ doping. RSC Adv. 6, 113469–113477 (2016).
Kumar, A., Silva, J. C. G. E. d., Kumar, K., Swart, H. C. & Maurya, S. K. et al. Improvement in upconversion/downshifting luminescence of Gd2O3:Ho3+/Yb3+ phosphor through Ca2+/Zn2+ incorporation and optical thermometry studies. Mater. Res. Bull. 112, 28–37 (2019).
Cheng, Y., Sun, K. & Ge, P. Yb3+ and Er3+ co-doped ZnGa2O4:Cr3+ powder phosphors: Combining green up-conversion emission and red persistent luminescence. Opt. Mater. 83, 13–18 (2018).
Yadav, R. S., Dhoble, S. J. & Rai, S. B. Improved photon upconversion photoluminescence and intrinsic optical bistability from a rare earth co-doped lanthanum oxide phosphor via Bi3+ doping. New J. Chem. 42, 7272–7282 (2018).
He, Y., Zhao, M., Song, Y., Zhao, G. & Ai, X. Effect of Bi3+ on fluorescence properties of YPO4:Dy3+ phosphors synthesized by a modified chemical co-precipitation method. J. Lumin. 131, 1144–1148 (2011).
Yadav, R. S. & Rai, S. B. Surface analysis and enhanced photoluminescence via Bi3+ doping in a Tb3+ doped Y2O3 nano-phosphor under UV excitation. J. Alloys Compd. 700, 228–237 (2017).
Monika, Yadav, R. S., Bahadur, A. & Rai, S. B. Near-infrared light excited highly pure green upconversion photoluminescence and intrinsic optical bistability sensing in a Ho3+/Yb3+ co-doped ZnGa2O4 phosphor through Li+ doping. J. Phys. Chem. C. 124, 10117–10128 (2020).
Lojpur, V., Nikolic, M., Mancic, L., Milosevic, O. & Dramicanin, M. D. Y2O3: Yb, Tm and Y2O3: Yb, Ho powders for low-temperature thermometry based on up-conversion fluorescence. Ceram. Int. 39, 1129–1134 (2013).
Suo, H., Guo, C. & Li, T. Broad-scope thermometry based on dual-color modulation up-conversion phosphor Ba5Gd8Zn4O21:Er3+/Yb3+. J. Phys. Chem. C. 120, 2914–2924 (2016).
Pandey, A. & Rai, V. K. Colour emission tunability in Ho3+–Tm3+–Yb3+ co-doped Y2O3 upconverted phosphor. Appl. Phys. B 109, 611–616 (2012).
Upasani, M. Synthesis of Y3Al5O12: Eu and Y3Al5O12:Eu, Si phosphors by combustion method: Comparative investigations on the structural and spectral properties. J. Adv. Ceram. 5, 344–355 (2016).
Badawi, E. A., Abdel-Rahman, M. A., Mostafa, A. & Abdel-Rahman, M. Determination of the crystallite size and micro-strain by novel method from XRD profile. Appl. Phys. 2, 1–15 (2019).
Wang, X. et al. Morphology control, spectrum modification and extended optical applications of rare earth ions doped phosphors. Phys. Chem. Chem. Phys. 22, 15120–15162 (2020).
Feng, X. et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science 312, 1504–1508 (2006).
Wu, X., Zeng, H., Yu, Q., Fan, C., Ren, J., Yuan, S. & Sun, L. Controlled growth and up-conversion luminescence of Y2O2S: Er3+ phosphor with the addition of Bi2O3. RSC Adv. 2, 9660–9664 (2012).
Wood, D. L. & Tauc, J. Weak absorption tails in amorphous semiconductors. Phys. Rev. B 5, 3144–3151 (1972).
Pandey, A. & Rai, V. K. Improved luminescence and temperature sensing performance of Ho3+/Yb3+/Zn2+:Y2O3 phosphor. Dalton Trans. 42, 11005–11011 (2013).
Dey, R., Kumari, A., Soni, A. K. & Rai, V. K. CaMoO4: Ho3+/Yb3+/Mg2+ upconverting phosphor for application in lighting devices and optical temperature sensing. Sens. Actuat. B: Chem. 210, 581–588 (2015).
Mondal, M., Rai, V. K., Srivastava, C., Sarkar, S. & Akash, R. Enhanced frequency upconversion in Ho3+/Yb3+/Li+: YMoO4 nanophosphors for photonic and security ink applications. J. Appl. Phys. 120, 233101 (2016).
Yadav, R. S., Yadav, R. V., Bahadur, A. & Rai, S. B. Enhanced white light emission from Tm3+/Yb3+/Ho3+ co-doped Na4ZnW3O12 nano-crystalline phosphor via Li+ doping. RSC Adv. 6, 51768–51776 (2016).
Chen, D. et al. Modifying the size and shape of monodisperse bifunctional alkaline-earth fluoride nanocrystals through lanthanide doping. J. Am. Chem. Soc. 132, 9976–9978 (2010).
Dutta, S., Som, S. & Chen TM. Promising Er3+/Yb3+ codoped GdBiW2O9 phosphor for temperature sensing by upconversion luminescence. ACS Omega 3, 11088–11096 (2018).
Li, L. et al. Unusual concentration induced anti thermal quenching of the Bi2+ emission from Sr2P2O7:Bi2+. Inorg. Chem. 54, 6028–6034 (2015).
Xu, C. et al. Luminescence properties, energy transfer and multisite luminescence of Bi3+/Sm3+/Eu3+-coactivated Ca20Al26Mg3Si3O68 as a potential phosphor for white-light LEDs. RSC Adv. 6, 89984–89993 (2016).
Wang, X., Wang, Ye., Yu, J., Bu, Y. & Yan, X. Modifying phase, shape and optical thermometry of NaGdF4:2%Er3+ phosphors through Ca2+ doping. Opt. Express 26, 21950–21959 (2018).
Xu, W. et al. Optical temperature sensing through the upconversion luminescence from Ho3+/Yb3+ codoped CaWO4. Sens. Actuat. B: Chem. 188, 1096–1100 (2013).
Jiang, L., Xiao, S., Yang, X., Ding, J. & Dong, K. Enhancement of up-conversion luminescence in Zn2SiO4:Yb3+, Er3+ by co-doping with Li+ or Bi3+. Appl. Phys. B 107, 477–481 (2012).
Chai, X., Li, J., Wang, X., Li, Y. & Yao, Xi. Upconversion luminescence and temperature sensing properties of Ho3+/Yb3+ codoped ZnWO4 phosphors based on fluorescence intensity ratios. RSC Adv. 7, 40046–40052 (2017).
Bednarkiewicz, A., Marciniak, L., Carlos, L. D. & Jaque, D. Standardizing luminescence nanothermometry for biomedical applications. Nanoscale 12, 14405–14421 (2020).
Mahata, M. K., Koppe, T., Kumar, K., Hofsäss, H. & Vetter, U. Upconversion photoluminescence of Ho3+–Yb3+ doped barium titanate nanocrystallites: Optical tools for structural phase detection and temperature probing. Sci. Rep. 10(8775), 1–12 (2020).
Zheng, S. et al. Lanthanide-doped NaGdF4 core–shell nanoparticles for non-contact self-referencing temperature sensors. Nanoscale 6, 5675–5679 (2014).
Xu, W., Gao, X., Zheng, L., Zhang, Z. & Cao, W. Short-wavelength upconversion emissions in Ho3+/Yb3+ codoped glass ceramic and the optical thermometry behavior. Opt. Express. 20, 18127–18137 (2012).
Liu, W. et al. Upconversion luminescence and favorable temperature sensing performance of eulytite-type Sr3Y(PO4)3:Yb3+/Ln3+ phosphors (Ln=Ho, Er, Tm). Sci. Technol. Adv. Mater. 20, 949–963 (2019).
Haro-González, P. et al. Characterization of Er3+ and Nd3+ doped strontium barium niobate glass ceramic as temperature sensors. Opt. Mater. 33, 742–745 (2011).
Liu, S. et al. High sensitive Ln3+/Tm3+/Yb3+ (Ln3+ = Ho3+, Er3+) tri-doped Ba3Y4O9 upconverting optical thermometric materials based on diverse thermal response from non-thermally coupled energy levels. Ceram. Int. 45, 1–10 (2019).
Wang, X., Wang, Ye., Jin, L., Bu, Y., Yang, X. L. & Yan, X. Controlling optical temperature detection of Ca3Al2O6: Yb3+, Er3+ phosphors through doping. J. Alloys Compds. 773, 393–400. (2019)
Cai, P., Wang, X. & Seo, H. J. Excitation power dependent optical temperature behaviors in Mn4+ doped oxyfluoride Na2WO2F4. Phys. Chem. Chem. Phys. 20, 2028–2035 (2018).
Suo, H. et al. All-in-one thermometer-heater up-converting platform YF3: Yb3+, Tm3+ operating in the first biological window. J. Mater. Chem. C 5, 1501–1507 (2017).
Du, P., Luo, L. H., Yue, Q. Y. & Li, W. P. The simultaneous realization of high- and low-temperature thermometry in Er3+/Yb3+-codoped Y2O3 nanoparticles. Mater. Lett. 143, 209–211 (2015).
Dong, B. et al. Optical thermometry through infrared excited green upconversion emissions in Er3+-Yb3+ codoped Al2O3. Appl. Phys. Lett. 90, 181117 (2007).
Angiuli, F., Cavalli, E., Boutinaud, P. & Mahiou, R. Emission properties of Sm3+/Bi3+-doped YPO4 phosphors. J. Lumin. 135, 239–242 (2013).
Zhu, H. et al. Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes. Nat. Comm. 5(4312), 1–10 (2014).
Lin, Y.-C., Karlsson, M. & Bettinelli, M. Inorganic phosphor materials for lighting. Top Curr. Chem. (Cham) 21(374), 1–47 (2016).
Sharma, S., Brahme, N., Bisen, D. P. & Dewangan, P. Cool white light emission from Dy3+ activated alkaline alumino silicate phosphors. Opt. Express 26, 29495–29508 (2018).
Mo, F., Chen, P., Guan, A., Zhang, W. & Zhou, L. Enhancement of luminescence intensity and color purity of MgxZn1–xMoO4:Eu3+, Bi3+ phosphors. J. Rare Earths 33, 1064–1071 (2015).
Nair, G. B., Kumar, A., Swart, H. C. & Dhoble, S. J. Facile precipitation synthesis of green-emitting BaY2F8:Yb3+, Ho3+ upconverting phosphor. Ceram. Int. 45, 14205–14213 (2019).
Acknowledgements
Monika acknowledges to Council of Scientific and Industrial Research, India for providing financial assistance of Junior Research Fellowship (Grant No. 09/013(0826)/2018-EMR-I). The authors also wish to acknowledge the Central Instrument Facility, IIT (BHU), Varanasi for providing SEM and EDS measurement facilities.
Author information
Authors and Affiliations
Contributions
M. has synthesized the phosphor samples and wrote this manuscript. R.S.Y, A.R. and S.B.R. read the manuscript and gave the valuable suggestions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Monika, Yadav, R.S., Rai, A. et al. NIR light guided enhanced photoluminescence and temperature sensing in Ho3+/Yb3+/Bi3+ co-doped ZnGa2O4 phosphor. Sci Rep 11, 4148 (2021). https://doi.org/10.1038/s41598-021-83644-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-83644-9
This article is cited by
-
Preparation of ZnGa2O4 nanoflowers and their full-color luminescence properties
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.