Featured
-
-
Letter |
 An oxygenase that forms and deoxygenates toxic epoxide
An oxygenase that forms and deoxygenates toxic epoxide
The epoxidase PaaABCE, which converts phenylacetyl-CoA into its ring-1,2-epoxide, is shown to be also able to mediate the NADPH-dependent removal of that epoxide, ensuring that the intracellular concentrations of the toxic epoxide does not exceed a certain ‘acceptable’ concentration.
- Robin Teufel
- , Thorsten Friedrich
- & Georg Fuchs
-
News & Views |
 A division duet
A division duet
The orchestration of cell division requires a programme of events choreographed by protein modification. A study shows that the relative activity of a phosphatase enzyme towards its substrates imposes order during the final act of division.
- Curt Wittenberg
-
Letter |
 Subnanometre-resolution structure of the intact Thermus thermophilus H+-driven ATP synthase
Subnanometre-resolution structure of the intact Thermus thermophilus H+-driven ATP synthase
Insights into the rotary mechanism of the Thermus thermophilus ATP synthase are obtained using electron cryomicroscopy to determine its three-dimensional structure calculated to subnanometre resolution.
- Wilson C. Y. Lau
- & John L. Rubinstein
-
Letter |
 An unanticipated architecture of the 750-kDa α6β6 holoenzyme of 3-methylcrotonyl-CoA carboxylase
An unanticipated architecture of the 750-kDa α6β6 holoenzyme of 3-methylcrotonyl-CoA carboxylase
The crystal structure of Pseudomonas aeruginosa 3-methylcrotonyl-CoA carboxylase is determined and found to be markedly different from that of propionyl-CoA carboxylase.
- Christine S. Huang
- , Peng Ge
- & Liang Tong
-
Brief Communications Arising |
Phiel et al. reply
- Christopher J. Phiel
- , Christina A. Wilson
- & Peter S. Klein
-
Brief Communications Arising |
 GSK-3α/β kinases and amyloid production in vivo
GSK-3α/β kinases and amyloid production in vivo
- Tomasz Jaworski
- , Ilse Dewachter
- & Fred Van Leuven
-
Letter |
 Image-based genome-wide siRNA screen identifies selective autophagy factors
Image-based genome-wide siRNA screen identifies selective autophagy factors
- Anthony Orvedahl
- , Rhea Sumpter Jr.
- & Beth Levine
-
Letter |
 Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically
Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically
The crystal structure of the enzyme MCR from methanogenic archaea shows that it is very similar to that of methanotrophic archaea; the differences observed may tune the enzymes for their respective biological context within the sea mats.
- Seigo Shima
- , Martin Krueger
- & Ulrich Ermler
-
Letter |
 Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing
Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing
Piwi protein Miwi is shown to be a small RNA-guided RNase in mice; disrupting the catalytic activity of Miwi results in increased accumulation of LINE1 retrotransposon transcripts and male infertility.
- Michael Reuter
- , Philipp Berninger
- & Ramesh S. Pillai
-
Letter |
 Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia
Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia
- Christian M. Metallo
- , Paulo A. Gameiro
- & Gregory Stephanopoulos
-
Letter |
 Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour
Natural polymorphisms in C. elegans HECW-1 E3 ligase affect pathogen avoidance behaviour
Natural variation in hecw-1 encoding an E3 ubiquitin ligase influences the avoidance of pathogenic bacteria by Caenorhabditis elegans and forms a molecular basis for behavioural variation.
- Howard C. Chang
- , Jennifer Paek
- & Dennis H. Kim
-
News & Views |
 A heavyweight knocked out
A heavyweight knocked out
Caspase-1 is one of the main culprits behind sepsis, a form of systemic inflammation. The related enzyme caspase-11 is also involved, but the relative roles of the two proteins have been confusing, until now. See Letter p.117
- Douglas R. Green
-
Article |
 Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase
Structure and function of the AAA+ protein CbbX, a red-type Rubisco activase
- Oliver Mueller-Cajar
- , Mathias Stotz
- & Manajit Hayer-Hartl
-
Letter |
 Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase
Saccharomyces cerevisiae THI4p is a suicide thiamine thiazole synthase
- Abhishek Chatterjee
- , N. Dinuka Abeydeera
- & Tadhg P. Begley
-
Letter |
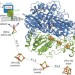 The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre
The crystal structure of an oxygen-tolerant hydrogenase uncovers a novel iron-sulphur centre
- Johannes Fritsch
- , Patrick Scheerer
- & Christian M. T. Spahn
-
Letter |
 Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1
Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1
- Valerie Garcia
- , Sarah E. L. Phelps
- & Matthew J. Neale
-
Letter |
 Active-site remodelling in the bifunctional fructose-1,6-bisphosphate aldolase/phosphatase
Active-site remodelling in the bifunctional fructose-1,6-bisphosphate aldolase/phosphatase
- Juan Du
- , Rafael F. Say
- & Oliver Einsle
-
Letter |
 ATP-induced helicase slippage reveals highly coordinated subunits
ATP-induced helicase slippage reveals highly coordinated subunits
- Bo Sun
- , Daniel S. Johnson
- & Michelle D. Wang
-
Letter |
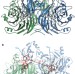 N2O binding at a [4Cu:2S] copper–sulphur cluster in nitrous oxide reductase
N2O binding at a [4Cu:2S] copper–sulphur cluster in nitrous oxide reductase
- Anja Pomowski
- , Walter G. Zumft
- & Oliver Einsle
-
Letter |
 Modulation of Rab GTPase function by a protein phosphocholine transferase
Modulation of Rab GTPase function by a protein phosphocholine transferase
- Shaeri Mukherjee
- , Xiaoyun Liu
- & Craig R. Roy
-
Article |
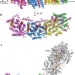 Structure of the membrane domain of respiratory complex I
Structure of the membrane domain of respiratory complex I
- Rouslan G. Efremov
- & Leonid A. Sazanov
-
Article |
 Type VI secretion delivers bacteriolytic effectors to target cells
Type VI secretion delivers bacteriolytic effectors to target cells
- Alistair B. Russell
- , Rachel D. Hood
- & Joseph D. Mougous
-
-
Feature |
 Chemistry: Enzyme expertise
Chemistry: Enzyme expertise
Biocatalysis specialists are in high demand in industry.
- Katharine Sanderson
-
Letter |
 Structure of mammalian AMPK and its regulation by ADP
Structure of mammalian AMPK and its regulation by ADP
- Bing Xiao
- , Matthew J. Sanders
- & Steven J. Gamblin
-
Letter |
 Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair
Crucial role for DNA ligase III in mitochondria but not in Xrcc1-dependent repair
Eukaryotic cells have several DNA ligases. DNA ligase III (Lig3) forms a complex with Xrcc1 that can function in nuclear repair. But, Lig3 null animals cannot be made; is this nuclear role in base excision repair its critical function? This is one of two papers showing that the role of Lig3 in the nucleus is non-essential. Rather, the catalytic activity of Lig3, but not Xrcc1, is essential for the maintenance of mitochondria.
- Deniz Simsek
- , Amy Furda
- & Maria Jasin
-
-
News & Views |
 The expanding arena of DNA repair
The expanding arena of DNA repair
The protein Sae2 mediates the repair of double-strand breaks in DNA. It emerges that Sae2 activity is controlled by both its modification with acetyl groups and its degradation by the process of autophagy. See Article p.74
- Catherine J. Potenski
- & Hannah L. Klein
-
Research Highlights |
Neuroscience: Enzyme helps pain persist
-
Letter |
 Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells
Lkb1 regulates quiescence and metabolic homeostasis of haematopoietic stem cells
Haematopoietic stem cells (HSCs) are very sensitive to energetic and oxidative stress, and modulation of the balance between their quiescence and proliferation is needed to respond to metabolic stress while preserving HSCs' long-term regenerative capacity. Here, and in two accompanying studies, it is shown that the tumour suppressor Lkb1 has a crucial role in maintaining energy homeostasis in haematopoietic cells.
- Boyi Gan
- , Jian Hu
- & Ronald A. DePinho
-
Article |
 Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA
Structure of a bacterial ribonuclease P holoenzyme in complex with tRNA
tRNAs are synthesized in a premature form that requires trimming of the 5′ and 3′ ends and modification of specific nucleotides. RNase P, a complex containing a long catalytic RNA and a protein cofactor, catalyses the cleavage that generates the mature 5′ end. Here, the structure of RNase P bound to mature tRNAPhe is solved. Recognition of the leader sequence and its mechanism of cleavage is determined by soaking an oligonucleotide corresponding to the premature 5′ end into the crystal.
- Nicholas J. Reiter
- , Amy Osterman
- & Alfonso Mondragón
-
News |
 High hopes for arthritis drugs
High hopes for arthritis drugs
Race is on to develop treatments that inhibit signalling proteins.
- Heidi Ledford
-
Letter |
 Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase
Iron-catalysed oxidation intermediates captured in a DNA repair dioxygenase
Mononuclear iron-containing oxygenases have many important roles in the cell, including the demethylation of DNA and histones. These authors crystallized the AlkB oxygenase in complex with various modified DNAs. By growing the crystals under anaerobic conditions and then exposing them to dioxygen to initiate oxidation, two different intermediates were trapped. A third type of intermediate was determined using additional computational analysis. These structures provide insight into how these enzymes perform oxidative demethylation.
- Chengqi Yi
- , Guifang Jia
- & Chuan He
-
Letter |
 Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme
Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme
These authors identify the human enzyme responsible for menaquinone-4 biosynthesis, a naturally occurring form of vitamin K. They find that UbiA prenyltransferase containing 1, a human homologue of a prenyltransferase gene from Escherichia coli, encodes an enzyme that can convert vitamin K derivatives into menaquinone-4.
- Kimie Nakagawa
- , Yoshihisa Hirota
- & Toshio Okano
-
Letter |
 Telomeres avoid end detection by severing the checkpoint signal transduction pathway
Telomeres avoid end detection by severing the checkpoint signal transduction pathway
The ends of chromosomes, known as telomeres, look like ends generated by double-strand breaks, but if treated as such the DNA damage repair system would initiate a checkpoint response and cause telomere–telomere fusions. These authors show that telomeres lack two types of histone modification that are required for recruitment of Crb2b53BP1, without which the checkpoint cannot be activated even if other DNA damage response proteins are recruited to a Taz1-deficient telomere.
- Tiago Carneiro
- , Lyne Khair
- & Miguel Godinho Ferreira
-
Letter |
 Crystal structure of the α6β6 holoenzyme of propionyl-coenzyme A carboxylase
Crystal structure of the α6β6 holoenzyme of propionyl-coenzyme A carboxylase
Propionyl-coenzyme A carboxylase (PCC) is a biotin-dependent enzyme that is essential for the catabolism of several amino acids, cholesterol and some fatty acids. Here, the crystal structure of a bacterial PCC is presented, along with a cryo-electron microscopy reconstruction showing a similar structure for human PCC. The structural information establishes a molecular basis for understanding the known disease-causing mutations in PCC, and is relevant to the holoenzymes of other biotin-dependent carboxylases.
- Christine S. Huang
- , Kianoush Sadre-Bazzaz
- & Liang Tong
-
News & Views |
 Blocking ubiquitin transfer
Blocking ubiquitin transfer
The protein OTUB1 inhibits DNA repair without using its enzymatic activity. Instead, it sequesters a protein that is required for the assembly of certain forms of ubiquitin chain, which function as key signals during repair.
- April Rose
- & Christian Schlieker
-
Letter |
 Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase
Dynamics and mechanism of repair of ultraviolet-induced (6–4) photoproduct by photolyase
The repair enzyme (6–4) photolyase uses light energy to cleave the ultraviolet-induced bond between pyrimidine dimers. These authors use ultrafast spectroscopy to examine the detailed electron and proton movements during the catalytic photocycle. Histidine 364 is identified as the crucial residue involved in the rate-limiting step.
- Jiang Li
- , Zheyun Liu
- & Dongping Zhong
-
Article |
 Structure and mechanism of human DNA polymerase η
Structure and mechanism of human DNA polymerase η
Ultraviolet radiation causes damage to DNA in skin cells, blocking DNA replication and causing mutations that can lead to cancer. One way in which the cell deals with such damage involves specialized DNA polymerases, such as Polη, that can bypass lesions. Here the crystal structure of Polη is presented at four consecutive steps during DNA synthesis through thymine dimers. Polη acts like a 'molecular splint' to stabilize damaged DNA, and accommodates the thymine dimer in an atypically large active site.
- Christian Biertümpfel
- , Ye Zhao
- & Wei Yang
-
News & Views |
 How to accurately bypass damage
How to accurately bypass damage
Ultraviolet radiation can cause cancer through DNA damage — specifically, by linking adjacent thymine bases. Crystal structures show how the enzyme DNA polymerase η accurately bypasses such lesions, offering protection.
- Suse Broyde
- & Dinshaw J. Patel
-
News & Views |
 A radically different enzyme
A radically different enzyme
The enzyme co-substrate S-adenosylmethionine is a potential source of two different free radicals, yet only one seemed to occur in nature. The discovery of an unusual enzyme reveals that both radicals can be formed.
- Joan B. Broderick
-
Letter |
 Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans
Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans
It is shown here that the methylation of histone proteins regulates lifespan in Caenorhabditis elegans. Deficiencies in members of the ASH-2 complex, which trimethylates histone H3 at lysine 4 (H3K4), extend worm lifespan. Meanwhile, the H3K4 demethylase RBR-2 is required for normal lifespan. These findings are consistent with the idea that an excess of H3K4 trimethylation reduces longevity. The extension of lifespan caused by ASH-2 deficiency requires an intact adult germline and the continuous production of mature eggs.
- Eric L. Greer
- , Travis J. Maures
- & Anne Brunet
-
Letter |
 A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases
A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases
Topoisomerases are enzymes that transiently make breaks in DNA, to prevent the build-up of topological stress and tangles as the genome is transcribed, replicated or repaired. Type II topoisomerases have been postulated to use a two-metal mechanism to break duplex DNA. Now, the structure of the DNA-binding and cleavage core of a yeast type II topoisomerase has been solved, showing that the enzyme uses a variation of the classical mechanism, and can also carry out the type of cleavage performed by type IA topoisomerases.
- Bryan H. Schmidt
- , Alex B. Burgin
- & James M. Berger
-
Article |
 The architecture of respiratory complex I
The architecture of respiratory complex I
Complex I is an enzyme of the respiratory chain, and is crucial to cellular energy production: it couples electron transfer between NADH and quinine to proton translocation. Here, structures are presented of the membrane domain of complex I from Escherichia coli, and of the entire complex I from Thermus thermophilus. It is proposed that conformational changes at the interface of the two main domains drive a particular 110-Å-long a-helix in a piston-like motion, tilting nearby transmembrane helices and causing proton translocation.
- Rouslan G. Efremov
- , Rozbeh Baradaran
- & Leonid A. Sazanov
-
News & Views |
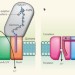 Piston drives a proton pump
Piston drives a proton pump
The membrane-spanning enzyme known as complex I couples the movement of electrons to that of protons as a way of converting energy. Crystal structures suggest how electron transfer drives proton pumping from afar.
- Tomoko Ohnishi
-
Letter |
 Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma
Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma
Toxoplasma gondii is an opportunistic human pathogen that secretes organelles called micronemes during infection. This is important for parasite motility, host-cell invasion and egress. It is now shown that the secretion of micronemes is dependent on the T. gondii calcium-dependent protein kinase 1. This kinase is not found in the parasite's mammalian hosts, and might represent a valid drug target.
- Sebastian Lourido
- , Joel Shuman
- & L. David Sibley
-
Letter |
 The PtdIns(3,4)P2 phosphatase INPP4A is a suppressor of excitotoxic neuronal death
The PtdIns(3,4)P2 phosphatase INPP4A is a suppressor of excitotoxic neuronal death
The enzyme inositol polyphosphate phosphatase 4A (INPP4A) removes phosphate groups from phosphatidylinositol-3,4-bisphosphate, a key cellular lipid. Here, a crucial role for INPP4A in maintaining the integrity of the brain is described. Mice that lack this enzyme suffer from neurodegeneration in the striatum of the brain, as well as severe involuntary movements. When present, INPP4A protects neurons from a particular type of cell death.
- Junko Sasaki
- , Satoshi Kofuji
- & Takehiko Sasaki
-
Letter |
 X-ray crystal structure of the light-independent protochlorophyllide reductase
X-ray crystal structure of the light-independent protochlorophyllide reductase
The ability of plants to 'green' in the dark is attributed to the activity of the dark-operative protochlorophyllide oxidoreductase (DPOR). This enzyme catalyses the stereospecific reduction of the C17≡C18 double bond of protochlorophyllide to form chlorophyllide a, the direct precursor of chlorophyll a. The X-ray crystal structure of the catalytic component of DPOR has now been solved. A chemical mechanism is proposed by which the reduction of the double bond may occur.
- Norifumi Muraki
- , Jiro Nomata
- & Yuichi Fujita
-
Letter |
 Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB
Histone H2A deubiquitinase activity of the Polycomb repressive complex PR-DUB
Polycomb group (PcG) proteins are transcriptional repressors that modify chromatin and regulate important developmental genes. One PcG-associated, chromatin-modifying activity is an enzyme that ubiquitinates histone H2A of chromatin. Here, a fruitfly PcG complex that is associated with H2A deubiquitination, and thereby with gene repression, is identified. PcG-mediated gene silencing might thus involve a dynamic balance between ubiquitination and deubiquitination of H2A.
- Johanna C. Scheuermann
- , Andrés Gaytán de Ayala Alonso
- & Jürg Müller

 Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes
Telomerase RNA biogenesis involves sequential binding by Sm and Lsm complexes The crystal structure of GXGD membrane protease FlaK
The crystal structure of GXGD membrane protease FlaK Enzyme can strengthen memories
Enzyme can strengthen memories