Featured
-
-
-
Review Article |
 The global burden of lung cancer: current status and future trends
The global burden of lung cancer: current status and future trends
Lung cancer is the commonest cancer globally. Reflecting patterns of smoking and other risk factor exposures, both the incidence of and mortality from lung cancer are highest in economically developed countries. Nonetheless, developing and less economically developed countries are likely to have the biggest increases in lung cancer in the coming years. In this Review, the authors describe the global epidemiology of lung cancer, and how changes in exposures, socioeconomic status, public health interventions and better treatment strategies are influencing both the incidence of and mortality from lung cancer.
- Amanda Leiter
- , Rajwanth R. Veluswamy
- & Juan P. Wisnivesky
-
News & Views |
A new era for glioma therapy — targeting mutant IDH
Hotspot point mutations in IDH1 occur in the vast majority of adult grade 2–3 gliomas. The understanding of their role in tumour biology continues to evolve. Therapeutic targeting of mutant IDH1 with vorasidenib demonstrated highly encouraging efficacy and minimal toxicity in a recent, randomized phase III trial involving patients with low-grade gliomas.
- David A. Reardon
- & Daniel P. Cahill
-
Comment |
Problematic crossovers in cancer drug trials
Crossover in a randomized trial can skew the interpretation of the efficacy of a cancer drug. In this Comment, I use examples from clinical trials presented at the 2023 ASCO annual meeting to highlight why ‘allowing’ crossover in randomized trials testing cancer drugs is problematic, and propose that crossovers should either be mandated or prohibited depending on the context.
- Bishal Gyawali
-
Review Article |
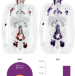 Quantitative PET-based biomarkers in lymphoma: getting ready for primetime
Quantitative PET-based biomarkers in lymphoma: getting ready for primetime
Despite advances in drug development for patients with lymphoma over the past decades, the identification of biomarkers for treatment selection remains an unmet need. The authors of this Review provide an overview of quantitative PET-based biomarkers in this patient population and discuss the challenges and opportunities in the integration of these biomarkers in clinical trials and the routine management of patients with lymphoma.
- Juan Pablo Alderuccio
- , Russ A. Kuker
- & Craig H. Moskowitz
-
News & Views |
 A critical appraisal of the ATLAS trial of maintenance therapy for multiple myeloma: end points, censoring and equipoise
A critical appraisal of the ATLAS trial of maintenance therapy for multiple myeloma: end points, censoring and equipoise
A recent report from the ATLAS trial comparing different maintenance strategies following haematopoeitic stem cell transplantation for multiple myeloma provides an opportunity to explore various themes of critical appraisal, including end points, the equipoise of trial design, and the part censoring can play in the validity of results.
- Ghulam Rehman Mohyuddin
- & Tomer Meirson
-
-
Review Article |
 Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy
Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy
Many studies attempting to identify biomarkers for predicting of immune-checkpoint inhibitor (ICI) efficacy have led to the description of Gut OncoMicrobiome Signatures (GOMS). Several GOMS support an association between oncogenesis and intestinal dysbiosis, and other GOMS are shared between patients with several cancer subtypes and individuals with seemingly unrelated chronic inflammatory disorders. The authors of this Review discuss these patterns as well as the findings from a meta-analysis of GOMS associated with clinical benefit from ICIs, and propose practical guidelines to incorporate GOMS in decision-making in immuno-oncology.
- Andrew Maltez Thomas
- , Marine Fidelle
- & Laurence Zitvogel
-
News & Views |
A triple dose of optimism: an initial foray into triplet therapy in metastatic renal cell carcinoma
COSMIC-313 combines all of the approved drugs against actionable targets in renal cell carcinoma into one triplet regimen. Although this approach has greater clinical efficacy than one of the standard-of-care doublet therapies, toxicities can limit adequate drug administration and, thus, we argue that this regimen should not yet be adopted. We also discuss ongoing investigations of other triplet regimens.
- Kathryn E. Beckermann
- & Brian I. Rini
-
Review Article |
 Antigen presentation in cancer — mechanisms and clinical implications for immunotherapy
Antigen presentation in cancer — mechanisms and clinical implications for immunotherapy
Immune-checkpoint inhibitors (ICIs) and other immunotherapies have revolutionized the treatment of patients with cancer. Nonetheless, most patients do not derive durable benefit, indicating a need for biomarkers to guide treatment selection. In this Review, the authors describe the role of antigen presentation in response to ICIs and other immunotherapies, with a focus on the role of molecular and/or genomic alterations affecting antigen presentation.
- Kailin Yang
- , Ahmed Halima
- & Timothy A. Chan
-
-
-
-
-
Review Article |
 Optimizing the safety of antibody–drug conjugates for patients with solid tumours
Optimizing the safety of antibody–drug conjugates for patients with solid tumours
Advances in technology have enabled the development of several novel antibody–drug conjugates (ADCs) with encouraging clinical activity in patients with advanced-stage solid tumours. Indications for these therapies are expanding rapidly to earlier lines of therapy. Nonetheless, the toxicities of these various agents are not trivial and can be fatal, even in patients with early stage disease. In this Review, the authors summarize the toxicities of ADCs in patients with solid tumours both as monotherapies and in combination with other agents and discuss various ongoing research efforts attempting to optimize the therapeutic index of these agents.
- Paolo Tarantino
- , Biagio Ricciuti
- & Sara M. Tolaney
-
Review Article |
 Emerging evidence for adapting radiotherapy to immunotherapy
Emerging evidence for adapting radiotherapy to immunotherapy
Radiotherapy has several key attributes that make it an attractive combination partner for immunotherapy; however, numerous clinical trials investigating the combination of these two treatment modalities have failed to demonstrate clear improvements in patient outcomes. In this Review, Galluzzi and colleagues discuss the evidence indicating that radiotherapy administered according to standard schedules and target volumes might impair immune fitness and, therefore, propose that adaptation of the radiotherapy regimens to immunotherapy (and not vice versa) might synergistically enhance the antitumour immune response to achieve meaningful clinical benefits.
- Lorenzo Galluzzi
- , Molykutty J. Aryankalayil
- & Silvia C. Formenti
-
Perspective |
 Circulating tumour cells for early detection of clinically relevant cancer
Circulating tumour cells for early detection of clinically relevant cancer
The authors of this Perspective propose that, with further improvement in detection efficiency, circulating tumour cells (CTCs), which are released early during cancer development, have the potential to be used for the early detection of clinically relevant, aggressive cancers. Thus, use of CTCs as diagnostic biomarkers might improve outcomes by enabling the identification of cancers at a stage at which they are more amenable to treatment while avoiding overtreatment of patients with indolent tumours.
- Rachel Lawrence
- , Melissa Watters
- & Yong-Jie Lu
-
Review Article |
 Early stage gastric adenocarcinoma: clinical and molecular landscapes
Early stage gastric adenocarcinoma: clinical and molecular landscapes
Long-term survival rates of patients with gastric cancer remain low, particularly in Western countries. This lack of progress, among other aspects, is likely to reflect a focus on empirical approaches that fail to account for the heterogeneity of gastric cancers. In this Review, the authors summarize the available evidence on the management of patients with early stage gastric cancers, with an emphasis on understanding the underlying biology in order to improve the outcomes in patients with these historically difficult-to-treat tumours.
- Yuki Hirata
- , Ayesha Noorani
- & Jaffer A. Ajani
-
Comment |
The FDA’s latest draft guidance on accelerated approvals — one step forward, two steps back?
The FDA Accelerated Approval pathway, which has been pivotal in enabling early access to new oncology drugs over the past three decades, has recently come under increased scrutiny. New draft guidance published in March 2023 offers several possible solutions to improve this pathway, with the ultimate goal of improving outcomes for patients with cancer, but might also have important limitations.
- David J. Benjamin
- & Mark P. Lythgoe
-
-
Research Highlight |
Venetoclax–obinutuzumab combinations are effective in fit patients with CLL
- Diana Romero
-
Review Article |
 Mitigating long-term and delayed adverse events associated with cancer treatment: implications for survivorship
Mitigating long-term and delayed adverse events associated with cancer treatment: implications for survivorship
The effective management of treatment-related events remains an unmet need in oncology. The authors of this Review discuss the underlying biological mechanisms, risk factors, most commonly used pharmacological and non-pharmacological management strategies, and clinical practice guidelines for the most common long-term (continuing beyond treatment) and late or delayed (following treatment) adverse events associated with chemotherapy and other anticancer treatments.
- Maryam B. Lustberg
- , Nicole M. Kuderer
- & Gary H. Lyman
-
Review Article |
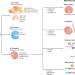 The roles and implications of RNA m6A modification in cancer
The roles and implications of RNA m6A modification in cancer
Dysregulation of N6-methyladenosine (m6A), the most prevalent internal modification in eukaryotic mRNA, is common in various cancer types. The authors of this Review provide an overview of the mechanisms of m6A-dependent RNA regulation, summarize current knowledge of their pathological effects and potential utility as biomarkers in cancer, and describe ongoing efforts to develop small-molecule inhibitors of oncogenic m6A modifiers.
- Xiaolan Deng
- , Ying Qing
- & Jianjun Chen
-
-
Review Article |
 Cholangiocarcinoma — novel biological insights and therapeutic strategies
Cholangiocarcinoma — novel biological insights and therapeutic strategies
Cholangiocarcinoma is a malignancy that continues to be associated with a dismal prognosis, and a better understanding of the disease biology is required to improve early detection and treatment strategies. In this Review, the authors describe key scientific and clinical advances made in this area over the past 5 years, encompassing novel insights into the tumour stroma and immune microenvironment, promising progress in developing liquid biopsy approaches for diagnosis and monitoring, clinical translation of molecularly targeted therapies, emerging immunotherapies and reassessment of the potential role of liver transplantation.
- Sumera I. Ilyas
- , Silvia Affo
- & Gregory J. Gores
-
-
News & Views |
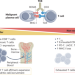 Determinants of response and resistance to T cell-engaging therapies in multiple myeloma
Determinants of response and resistance to T cell-engaging therapies in multiple myeloma
Bispecific T cell engagers offer a novel treatment approach for patients with multiple myeloma, although mechanisms of resistance are largely unknown. Here, we discuss the implications of a recent report from Friedrich et al. that highlights the importance of pre-treatment T cell characteristics for a response to the T cell engager elranatamab and how these data might be used to inform future study and trial design.
- Shonali Midha
- & Kenneth C. Anderson
-
Comment |
 National value-based pricing negotiation for oncology drugs — lessons from China
National value-based pricing negotiation for oncology drugs — lessons from China
Between 2016 and 2022, 83 previously approved oncology drugs were covered and reimbursed in China through a value-based pricing negotiation programme, which resulted in substantial price cuts but did not improve the correlation between drug cost and clinical benefit. Herein, we call for an improved, transparent value-based pricing model to better account for high-value innovation in oncology drugs.
- Jing Yuan
- , Minghui Li
- & Z. Kevin Lu
-
-
-
Review Article |
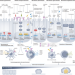 Gut microbiota in colorectal cancer development and therapy
Gut microbiota in colorectal cancer development and therapy
Emerging data indicate a central role for the microbiota in all aspects of colorectal cancer (CRC). Despite this general consensus, understanding the role of specific components of the microbiota in such a way that enables the development of clinical interventions or tools to inform clinical decision-making has thus far proved challenging. In this Review, the authors summarize the role of the microbiota in CRC, including in prevention, in interactions with treatment and as a source of novel biomarkers.
- Chi Chun Wong
- & Jun Yu
-
-
Review Article |
 Personalizing neoadjuvant immune-checkpoint inhibition in patients with melanoma
Personalizing neoadjuvant immune-checkpoint inhibition in patients with melanoma
Neoadjuvant immune-checkpoint inhibition is a promising emerging treatment strategy that potentially enables patients with a good response to initial therapy to avoid further treatment and the associated toxicity risks, while also identifying those who might require treatment escalation. In this Review, the authors describe treatment personalization strategies based on the initial response to one or more neoadjuvant immune-checkpoint inhibitors and consider the potential to expand this approach beyond patients with melanoma.
- Minke W. Lucas
- , Judith M. Versluis
- & Christian U. Blank
-
-
-
-
Review Article |
 Improving outcomes in patients with oesophageal cancer
Improving outcomes in patients with oesophageal cancer
Oesophageal cancer is one of the most common malignancies worldwide and is associated with considerable morbidity and mortality. In this Review, the authors highlight advances made across the disease continuum that have improved the management and outcomes of patients with oesophageal cancer. These advances include an increased understanding of the disease biology, improvements in screening, the development of minimally invasive endoscopic monitoring and management technologies, refinement of surgical techniques and perioperative management, and novel radiotherapy and systemic therapy approaches. Continual multidisciplinary efforts across all these aspects of care will further improve patient outcomes.
- Manish A. Shah
- , Nasser Altorki
- & Julian A. Abrams
-
Review Article |
 Understanding the activity of antibody–drug conjugates in primary and secondary brain tumours
Understanding the activity of antibody–drug conjugates in primary and secondary brain tumours
Antibody–drug conjugates (ADCs) have demonstrated efficacy in patients with various cancers, although their antitumour activity in the central nervous system (CNS) might be limited by the blood–brain barrier. In this Review, the authors describe the available clinical data emphasizing the heterogeneous activity of ADCs against primary or secondary brain tumours and ongoing clinical trials in this area. In addition, they discuss physical, biological and molecular determinants of the CNS activity of ADCs, as well as potential strategies to improve delivery of these agents to brain tumours.
- Maximilian J. Mair
- , Rupert Bartsch
- & Matthias Preusser
-
-
Review Article |
 Long-term outcomes following CAR T cell therapy: what we know so far
Long-term outcomes following CAR T cell therapy: what we know so far
Chimeric antigen receptor (CAR) T cells have dramatically improved the outcomes of patients with certain relapsed and/or refractory haematological malignancies. Owing to the promising short-term survival outcomes achieved, long-term data on both safety and survival are becoming increasingly relevant. In this Review, the authors describe the available long-term follow-up data from early studies testing the safety and efficacy of receiving CAR T cells targeting CD19 as well as more recent data on BCMA-targeted CAR T cells in patients with relapsed and/or refractory multiple myeloma.
- Kathryn M. Cappell
- & James N. Kochenderfer
-
Comment |
Envisioning trans-inclusive and trans-specific cancer care
Transgender patients are a marginalized group for whom current standards of oncology have yet to be optimized. In this Comment, we highlight opportunities for transgender-inclusive and transgender-specific practices across the cancer care continuum and identify evidence gaps that will need to be filled to attain optimal care for transgender populations.
- Elle Lett
- , Joannie M. Ivory
- & Mya L. Roberson
-
-
Comment |
Navigating approval pathways for immunotherapy in NSCLC: should criteria be revised?
Recent FDA reviews of cemiplimab and sintilimab combined with chemotherapy for patients with advanced-stage non-small-cell lung cancer reached discordant outcomes, as cemiplimab was approved and sintilimab was rejected. The applications share many serious faults, including neither serving an unmet need nor enrolling any patients from the USA. We argue that the FDA criteria should be more transparent and consistent; moreover, the historical policy of the FDA to abstain from consideration of the cost of a drug perpetuates a crisis in oncology care and should be re-examined.
- Aakash Desai
- , Caleb J. Smith
- & Howard Jack West
-
News & Views |
Combination immunomodulation for immune-checkpoint-inhibitor-associated myocarditis
Immune-checkpoint-inhibitor-associated myocarditis has a high fatality rate, warranting the development of more-effective treatment strategies. Herein, we discuss a recent report of a series of patients who were managed using a novel approach that involved personalized abatacept dosing, ruxolitinib and close respiratory monitoring, which was associated with low mortality.
- Douglas B. Johnson
- & Alexander M. Menzies
-
Research Highlight |
 Adding immune-checkpoint inhibitors to chemotherapy extends survival in endometrial cancer
Adding immune-checkpoint inhibitors to chemotherapy extends survival in endometrial cancer
- Diana Romero
-
-
-
-
Review Article |
 Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention
Global burden of gastric cancer: epidemiological trends, risk factors, screening and prevention
Globally, gastric cancer is a common and highly fatal cancer with two anatomical subtypes, non-cardia and cardia gastric cancer. Helicobacter pylori causes almost 90% of distal gastric cancers worldwide. The authors of this Review summarize the current epidemiology of gastric cancer and the evidence and implications of primary and secondary prevention efforts.
- Aaron P. Thrift
- , Theresa Nguyen Wenker
- & Hashem B. El-Serag
Browse broader subjects
Browse narrower subjects
- Bone cancer
- Breast cancer
- Cancer epidemiology
- Cancer genetics
- Cancer genomics
- Cancer imaging
- Cancer metabolism
- Cancer microenvironment
- Cancer models
- Cancer of unknown primary
- Cancer prevention
- Cancer screening
- Cancer stem cells
- Cancer therapy
- CNS cancer
- Cysts
- Embryonal neoplasms
- Endocrine cancer
- Eye cancer
- Gastrointestinal cancer
- Germ cell tumours
- Gynaecological cancer
- Haematological cancer
- Hamartoma
- Head and neck cancer
- Lung cancer
- Mesothelioma
- Metastasis
- Oncogenes
- Oral cancer
- Paediatric cancer
- Sarcoma
- Skin cancer
- Testicular cancer
- Tumour angiogenesis
- Tumour biomarkers
- Tumour heterogeneity
- Tumour immunology
- Tumour-suppressor proteins
- Tumour virus infections
- Urological cancer

 Immune-checkpoint inhibition for resectable non-small-cell lung cancer — opportunities and challenges
Immune-checkpoint inhibition for resectable non-small-cell lung cancer — opportunities and challenges From ASCO 2023
From ASCO 2023 ROAR brings in a new histology-agnostic approval for rare cancers
ROAR brings in a new histology-agnostic approval for rare cancers Mortality is similar with active monitoring
Mortality is similar with active monitoring