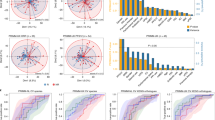
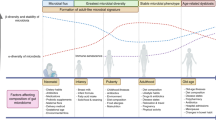
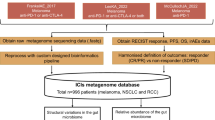
Abstract
Oncogenesis is associated with intestinal dysbiosis, and stool shotgun metagenomic sequencing in individuals with this condition might constitute a non-invasive approach for the early diagnosis of several cancer types. The prognostic relevance of antibiotic intake and gut microbiota composition urged investigators to develop tools for the detection of intestinal dysbiosis to enable patient stratification and microbiota-centred clinical interventions. Moreover, since the advent of immune-checkpoint inhibitors (ICIs) in oncology, the identification of biomarkers for predicting their efficacy before starting treatment has been an unmet medical need. Many previous studies addressing this question, including a meta-analysis described herein, have led to the description of Gut OncoMicrobiome Signatures (GOMS). In this Review, we discuss how patients with cancer across various subtypes share several GOMS with individuals with seemingly unrelated chronic inflammatory disorders who, in turn, tend to have GOMS different from those of healthy individuals. We discuss findings from the aforementioned meta-analysis of GOMS patterns associated with clinical benefit from or resistance to ICIs across different cancer types (in 808 patients), with a focus on metabolic and immunological surrogate markers of intestinal dysbiosis, and propose practical guidelines to incorporate GOMS in decision-making for prospective clinical trials in immuno-oncology.
Key points
-
Oncogenesis can cause a stress ileopathy (characterized by an ectopic accumulation of enteroendocrine cells and an imbalance between sympathetic and cholinergic signalling) associated with an intestinal dysbiosis.
-
Studies of the links between intestinal dysbiosis and microbial tissue colonization or infection could provide novel insights relevant to the aetiology, prevention and treatment of various cancer types, such as pancreatic adenocarcinomas and urothelial carcinomas.
-
Patients with cancer share gut microbiome signatures with individuals with seemingly unrelated disorders characterized by an imbalance between health-related and chronic inflammatory disease-related commensals.
-
In the past decade, investigators have identified Gut OncoMicrobiome signatures (GOMS) that share profile commonalities across cancer histotypes.
-
GOMS might constitute a promising, non-invasive and cost-effective approach for early diagnosis of various different cancer types.
-
GOMS are candidate predictors of resistance to immune-checkpoint inhibitors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hanahan, D. Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022).
Cassidy, L. D. et al. Temporal inhibition of autophagy reveals segmental reversal of ageing with increased cancer risk. Nat. Commun. 11, 307 (2020).
Demaria, M. et al. Cellular senescence promotes adverse effects of chemotherapy and cancer relapse. Cancer Discov. 7, 165–176 (2017).
López-Otín, C. & Kroemer, G. Hallmarks of health. Cell 184, 33–63 (2021).
Derosa, L. et al. The immuno-oncological challenge of COVID-19. Nat. Cancer 1, 946–964 (2020).
Rutkowski, M. R. et al. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell 27, 27–40 (2015).
McKee, A. M. et al. Antibiotic-induced disturbances of the gut microbiota result in accelerated breast tumor growth. iScience 24, 103012 (2021).
Buchta Rosean, C. et al. Preexisting commensal dysbiosis is a host-intrinsic regulator of tissue inflammation and tumor cell dissemination in hormone receptor-positive breast cancer. Cancer Res. 79, 3662–3675 (2019).
Ghosh, T. S., Das, M., Jeffery, I. B. & O’Toole, P. W. Adjusting for age improves identification of gut microbiome alterations in multiple diseases. eLife 9, e50240 (2020).
Wilmanski, T. et al. Gut microbiome pattern reflects healthy ageing and predicts survival in humans. Nat. Metab. 3, 274–286 (2021).
Bindels, L. B. et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget 9, 18224–18238 (2018).
Ubachs, J. et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 12, 2007–2021 (2021).
Zitvogel, L., Ma, Y., Raoult, D., Kroemer, G. & Gajewski, T. F. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science 359, 1366–1370 (2018).
Claesson, M. J. et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl Acad. Sci. USA 108, 4586–4591 (2011).
Roager, H. M. & Licht, T. R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 9, 3294 (2018).
Sonowal, R. et al. Indoles from commensal bacteria extend healthspan. Proc. Natl Acad. Sci. USA 114, E7506–E7515 (2017).
Alexeev, E. E. et al. Microbiota-derived indole metabolites promote human and murine intestinal homeostasis through regulation of interleukin-10 receptor. Am. J. Pathol. 188, 1183–1194 (2018).
Bindels, L. B. et al. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 10, 1456–1470 (2016).
Ma, C. et al. Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360, eaan5931 (2018).
Song, X. et al. Microbial bile acid metabolites modulate gut RORγ+ regulatory T cell homeostasis. Nature 577, 410–415 (2020).
Fidelle, M. et al. A microbiota-modulated checkpoint directs immunosuppressive intestinal T cells into cancers. Science 380, eabo2296 (2023).
Sato, Y. et al. Novel bile acid biosynthetic pathways are enriched in the microbiome of centenarians. Nature 599, 458–464 (2021).
Gacesa, R. et al. Environmental factors shaping the gut microbiome in a Dutch population. Nature 604, 732–739 (2022).
Jackson, M. A. et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 65, 749–756 (2016).
Yonekura, S. et al. Cancer induces a stress ileopathy depending on β-adrenergic receptors and promoting dysbiosis that contributes to carcinogenesis. Cancer Discov. 12, 1128–1151 (2022).
Derosa, L. et al. Gut bacteria composition drives primary resistance to cancer immunotherapy in renal cell carcinoma patients. Eur. Urol. 78, 195–206 (2020).
Terrisse, S. et al. Intestinal microbiota influences clinical outcome and side effects of early breast cancer treatment. Cell Death Differ. 28, 2778–2796 (2021).
Terrisse, S. et al. Immune system and intestinal microbiota determine efficacy of androgen deprivation therapy against prostate cancer. J. Immunother. Cancer 10, e004191 (2022).
Pernigoni, N. et al. Commensal bacteria promote endocrine resistance in prostate cancer through androgen biosynthesis. Science 374, 216–224 (2021).
Geller, L. T. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Collins, J. R. Small intestinal mucosal damage with villous atrophy: a review of the literature. Am. J. Clin. Pathol. 44, 36–44 (1965).
Gilat, T., Fischel, B., Danon, J. & Loewenthal, M. Morphology of small bowel mucosa in malignancy. Digestion 7, 147–155 (1972).
Stanley, D. et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284 (2016).
Singh, V. et al. Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440 (2016).
Lynch, S. V. & Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 375, 2369–2379 (2016).
Gupta, V. K. et al. A predictive index for health status using species-level gut microbiome profiling. Nat. Commun. 11, 4635 (2020).
Blanco-Míguez, A. et al. Extending and improving metagenomic taxonomic profiling with uncharacterized species using MetaPhlAn 4. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01688-w (2023)
Gopalakrishnan, V. et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103 (2018).
Lee, K. A. et al. Cross-cohort gut microbiome associations with immune checkpoint inhibitor response in advanced melanoma. Nat. Med. 28, 535–544 (2022).
McCulloch, J. A. et al. Intestinal microbiota signatures of clinical response and immune-related adverse events in melanoma patients treated with anti-PD-1. Nat. Med. 28, 545–556 (2022).
Derosa, L. et al. Intestinal Akkermansia muciniphila predicts clinical response to PD-1 blockade in patients with advanced non-small-cell lung cancer. Nat. Med. 28, 315–324 (2022).
Routy, B. et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Wind, T. T. et al. Gut microbial species and metabolic pathways associated with response to treatment with immune checkpoint inhibitors in metastatic melanoma. Melanoma Res. 30, 235–246 (2020).
Frankel, A. E. et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 19, 848–855 (2017).
Nagata, N. et al. Metagenomic identification of microbial signatures predicting pancreatic cancer from a multinational study. Gastroenterology 163, 222–238 (2022).
Yu, J. et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut 66, 70–78 (2017).
Feng, Q. et al. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nat. Commun. 6, 6528 (2015).
Yachida, S. et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat. Med. 25, 968–976 (2019).
Wirbel, J. et al. Meta-analysis of fecal metagenomes reveals global microbial signatures that are specific for colorectal cancer. Nat. Med. 25, 679–689 (2019).
Thomas, A. M. et al. Metagenomic analysis of colorectal cancer datasets identifies cross-cohort microbial diagnostic signatures and a link with choline degradation. Nat. Med. 25, 667–678 (2019).
Vogtmann, E. et al. Colorectal cancer and the human gut microbiome: reproducibility with whole-genome shotgun sequencing. PLoS ONE 11, e0155362 (2016).
Zeller, G. et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 10, 766 (2014).
Spencer, C. N. et al. Dietary fiber and probiotics influence the gut microbiome and melanoma immunotherapy response. Science 374, 1632–1640 (2021).
Kartal, E. et al. A faecal microbiota signature with high specificity for pancreatic cancer. Gut 71, 1359–1372 (2022).
Peters, B. A. et al. Relating the gut metagenome and metatranscriptome to immunotherapy responses in melanoma patients. Genome Med. 11, 61 (2019).
Asnicar, F. et al. Microbiome connections with host metabolism and habitual diet from 1,098 deeply phenotyped individuals. Nat. Med. 27, 321–332 (2021).
De Filippis, F. et al. Distinct genetic and functional traits of human intestinal Prevotella copri strains are associated with different habitual diets. Cell Host Microbe 25, 444–453.e3 (2019).
Dhakan, D. B. et al. The unique composition of Indian gut microbiome, gene catalogue, and associated fecal metabolome deciphered using multi-omics approaches. Gigascience 8, giz004 (2019).
Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Keohane, D. M. et al. Microbiome and health implications for ethnic minorities after enforced lifestyle changes. Nat. Med. 26, 1089–1095 (2020).
Zhernakova, A. et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 352, 565–569 (2016).
Vieira-Silva, S. et al. Statin therapy is associated with lower prevalence of gut microbiota dysbiosis. Nature 581, 310–315 (2020).
Nielsen, H. B. et al. Identification and assembly of genomes and genetic elements in complex metagenomic samples without using reference genomes. Nat. Biotechnol. 32, 822–828 (2014).
Qin, J. et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 (2012).
Qin, N. et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 (2014).
Schirmer, M. et al. Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1897 (2016).
Xie, H. et al. Shotgun metagenomics of 250 adult twins reveals genetic and environmental impacts on the gut microbiome. Cell Syst. 3, 572–584.e3 (2016).
Zeevi, D. et al. Personalized nutrition by prediction of glycemic responses. Cell 163, 1079–1094 (2015).
Nejman, D. et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science 368, 973–980 (2020).
Bogert, B., van den, Meijerink, M., Zoetendal, E. G., Wells, J. M. & Kleerebezem, M. Immunomodulatory properties of Streptococcus and Veillonella isolates from the human small intestine microbiota. PLoS ONE 9, e114277 (2014).
Hong, H.-E., Kim, A.-S., Kim, M.-R., Ko, H.-J. & Jung, M. K. Does the use of proton pump inhibitors increase the risk of pancreatic cancer? A systematic review and meta-analysis of epidemiologic studies. Cancers 12, 2220 (2020).
Zackular, J. P., Rogers, M. A. M., Ruffin, M. T. & Schloss, P. D. The human gut microbiome as a screening tool for colorectal cancer. Cancer Prev. Res. 7, 1112–1121 (2014).
Beghini, F. et al. Integrating taxonomic, functional, and strain-level profiling of diverse microbial communities with bioBakery 3. Elife 10, e65088 (2021).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Long, X. et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 4, 2319–2330 (2019).
Drewes, J. L. et al. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes 3, 34 (2017).
Flemer, B. et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 67, 1454–1463 (2018).
Schmidt, T. S. et al. Extensive transmission of microbes along the gastrointestinal tract. Elife 8, e42693 (2019).
Nakatsu, G. et al. Alterations in enteric virome are associated with colorectal cancer and survival outcomes. Gastroenterology 155, 529–541.e5 (2018).
Tomkovich, S. et al. Human colon mucosal biofilms from healthy or colon cancer hosts are carcinogenic. J. Clin. Invest. 129, 1699–1712 (2019).
Dejea, C. M. et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc. Natl Acad. Sci. USA 111, 18321–18326 (2014).
Lui, R. N. et al. Global increasing incidence of young-onset colorectal cancer across 5 continents: a joinpoint regression analysis of 1,922,167 cases. Cancer Epidemiol. Biomark. Prev. 28, 1275–1282 (2019).
Yang, Y. et al. Dysbiosis of human gut microbiome in young-onset colorectal cancer. Nat. Commun. 12, 6757 (2021).
Kong, C. et al. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut https://doi.org/10.1136/gutjnl-2022-327156 (2022).
Thomas, A. M. & Segata, N. Multiple levels of the unknown in microbiome research. BMC Biol. 17, 48 (2019).
Lin, Y. et al. Altered mycobiota signatures and enriched pathogenic Aspergillus rambellii are associated with colorectal cancer based on multicohort fecal metagenomic analyses. Gastroenterology 163, 908–921 (2022).
Liu, N.-N. et al. Multi-kingdom microbiota analyses identify bacterial-fungal interactions and biomarkers of colorectal cancer across cohorts. Nat. Microbiol. 7, 238–250 (2022).
Topalian, S. L., Taube, J. M. & Pardoll, D. M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science 367, eaax0182 (2020).
Matson, V., Chervin, C. S. & Gajewski, T. F. Cancer and the microbiome–influence of the commensal microbiota on cancer, immune responses, and immunotherapy. Gastroenterology 160, 600–613 (2021).
Derosa, L. et al. Microbiota-centered interventions: the next breakthrough in immuno-oncology? Cancer Discov. 11, 2396–2412 (2021).
Matson, V. et al. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108 (2018).
Hakozaki, T. et al. The gut microbiome associates with immune checkpoint inhibition outcomes in patients with advanced non-small cell lung cancer. Cancer Immunol. Res. 8, 1243–1250 (2020).
Cascone, T. et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat. Med. 27, 504–514 (2021).
Salgia, N. J. et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur. Urol. 78, 498–502 (2020).
Jin, Y. et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in chinese patients with NSCLC. J. Thorac. Oncol. 14, 1378–1389 (2019).
Newsome, R. C. et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 14, 35 (2022).
Park, E. M. et al. Targeting the gut and tumor microbiota in cancer. Nat. Med. 28, 690–703 (2022).
Mao, J. et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 9, e003334 (2021).
Peng, Z. et al. The gut microbiome is associated with clinical response to anti-PD-1/PD-L1 immunotherapy in gastrointestinal cancer. Cancer Immunol. Res. 8, 1251–1261 (2020).
Zheng, Y. et al. Gut microbiome affects the response to anti-PD-1 immunotherapy in patients with hepatocellular carcinoma. J. Immunother. Cancer 7, 193 (2019).
Depommier, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103 (2019).
de Vos, W. M., Tilg, H., Van Hul, M. & Cani, P. D. Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032 (2022).
Schneider, K. M. et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat. Commun. 13, 3964 (2022).
Ansaldo, E. et al. Akkermansia muciniphila induces intestinal adaptive immune responses during homeostasis. Science 364, 1179–1184 (2019).
Cascone, T. et al. Neoadjuvant chemotherapy plus nivolumab with or without ipilimumab in operable non-small cell lung cancer: the phase 2 platform NEOSTAR trial. Nat. Med. 29, 593–604 (2023).
Shaikh, F. Y. et al. Murine fecal microbiota transfer models selectively colonize human microbes and reveal transcriptional programs associated with response to neoadjuvant checkpoint inhibitors. Cancer Immunol. Immunother. 71, 2405–2420 (2022).
Shaikh, F. Y. et al. A uniform computational approach improved on existing pipelines to reveal microbiome biomarkers of nonresponse to immune checkpoint inhibitors. Clin. Cancer Res. 27, 2571–2583 (2021).
Limeta, A., Ji, B., Levin, M., Gatto, F. & Nielsen, J. Meta-analysis of the gut microbiota in predicting response to cancer immunotherapy in metastatic melanoma. JCI Insight 5, 140940 (2020).
He, Y. et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 24, 1532–1535 (2018).
Simpson, R. C. et al. Diet-driven microbial ecology underpins associations between cancer immunotherapy outcomes and the gut microbiome. Nat. Med. https://doi.org/10.1038/s41591-022-01965-2 (2022).
Iida, N. et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science 342, 967–970 (2013).
Hegazy, A. N. et al. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology 153, 1320–1337.e16 (2017).
Bourgonje, A. R. et al. Patients with inflammatory bowel disease show IgG immune responses towards specific intestinal bacterial genera. Front. Immunol. 13, 842911 (2022).
Lodes, M. J. et al. Bacterial flagellin is a dominant antigen in Crohn disease. J. Clin. Invest. 113, 1296–1306 (2004).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Rengarajan, S. et al. Dynamic immunoglobulin responses to gut bacteria during inflammatory bowel disease. Gut Microbes 11, 405–420 (2020).
Overacre-Delgoffe, A. E. et al. Microbiota-specific T follicular helper cells drive tertiary lymphoid structures and anti-tumor immunity against colorectal cancer. Immunity 54, 2812–2824.e4 (2021).
Noble, A. et al. Altered immunity to microbiota, B cell activation and depleted γδ/resident memory T cells in colorectal cancer. Cancer Immunol. Immunother. https://doi.org/10.1007/s00262-021-03135-8 (2022).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541.e5 (2022).
Goubet, A.-G. et al. Escherichia coli-specific CXCL13-producing TFH are associated with clinical efficacy of neoadjuvant PD-1 blockade against muscle-invasive bladder cancer. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-22-0201 (2022).
Wu, J. et al. A highly polarized TH2 bladder response to infection promotes epithelial repair at the expense of preventing new infections. Nat. Immunol. 21, 671–683 (2020).
Yacouba, A., Tidjani Alou, M., Lagier, J.-C., Dubourg, G. & Raoult, D. Urinary microbiota and bladder cancer: a systematic review and a focus on uropathogens. Semin. Cancer Biol. https://doi.org/10.1016/j.semcancer.2021.12.010 (2022).
Zitvogel, L. & Kroemer, G. Cross-reactivity between microbial and tumor antigens. Curr. Opin. Immunol. 75, 102171 (2022).
Fluckiger, A. et al. Cross-reactivity between tumor MHC class I-restricted antigens and an enterococcal bacteriophage. Science 369, 936–942 (2020).
Vétizou, M. et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 350, 1079–1084 (2015).
Daillère, R. et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity 45, 931–943 (2016).
Rong, Y. et al. Reactivity toward Bifidobacterium longum and Enterococcus hirae demonstrate robust CD8+ T cell response and better prognosis in HBV-related hepatocellular carcinoma. Exp. Cell Res. 358, 352–359 (2017).
Davar, D. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 371, 595–602 (2021).
Smith, M. et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 28, 713–723 (2022).
Whitfield-Cargile, C. M. et al. The non-invasive exfoliated transcriptome (exfoliome) reflects the tissue-level transcriptome in a mouse model of NSAID enteropathy. Sci. Rep. 7, 14687 (2017).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Haenen, D. et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine. J. Nutr. 143, 274–283 (2013).
Arpaia, N. et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455 (2013).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Park, J., Goergen, C. J., HogenEsch, H. & Kim, C. H. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J. Immunol. 196, 2388–2400 (2016).
Kespohl, M. et al. The microbial metabolite butyrate induces expression of Th1-associated factors in CD4+ T cells. Front. Immunol. 8, 1036 (2017).
Mariño, E. et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562 (2017).
Mathewson, N. D. et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513 (2016).
Trompette, A. et al. Dietary fiber confers protection against flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 48, 992–1005.e8 (2018).
Bachem, A. et al. Microbiota-derived short-chain fatty acids promote the memory potential of antigen-activated CD8+ T cells. Immunity 51, 285–297.e5 (2019).
Luu, M. et al. The short-chain fatty acid pentanoate suppresses autoimmunity by modulating the metabolic-epigenetic crosstalk in lymphocytes. Nat. Commun. 10, 760 (2019).
Yuille, S., Reichardt, N., Panda, S., Dunbar, H. & Mulder, I. E. Human gut bacteria as potent class I histone deacetylase inhibitors in vitro through production of butyric acid and valeric acid. PLoS ONE 13, e0201073 (2018).
Luu, M. et al. Microbial short-chain fatty acids modulate CD8+ T cell responses and improve adoptive immunotherapy for cancer. Nat. Commun. 12, 4077 (2021).
Nomura, M. et al. Association of short-chain fatty acids in the gut microbiome with clinical response to treatment with nivolumab or pembrolizumab in patients with solid cancer tumors. JAMA Netw. Open 3, e202895 (2020).
Coutzac, C. et al. Systemic short chain fatty acids limit antitumor effect of CTLA-4 blockade in hosts with cancer. Nat. Commun. 11, 2168 (2020).
Uribe-Herranz, M. et al. Gut microbiota modulate dendritic cell antigen presentation and radiotherapy-induced antitumor immune response. J. Clin. Invest. 130, 466–479 (2020).
Munn, D. H. et al. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J. Exp. Med. 189, 1363–1372 (1999).
Uyttenhove, C. et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat. Med. 9, 1269–1274 (2003).
Wang, Y., Hu, G.-F. & Wang, Z.-H. The status of immunosuppression in patients with stage IIIB or IV non-small-cell lung cancer correlates with the clinical characteristics and response to chemotherapy. Onco Targets Ther. 10, 3557–3566 (2017).
Holmgaard, R. B., Zamarin, D., Munn, D. H., Wolchok, J. D. & Allison, J. P. Indoleamine 2,3-dioxygenase is a critical resistance mechanism in antitumor T cell immunotherapy targeting CTLA-4. J. Exp. Med. 210, 1389–1402 (2013).
Botticelli, A. et al. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J. Transl. Med. 16, 219 (2018).
Li, H. et al. Metabolomic adaptations and correlates of survival to immune checkpoint blockade. Nat. Commun. 10, 4346 (2019).
Long, G. V. et al. Epacadostat plus pembrolizumab versus placebo plus pembrolizumab in patients with unresectable or metastatic melanoma (ECHO-301/KEYNOTE-252): a phase 3, randomised, double-blind study. Lancet Oncol. 20, 1083–1097 (2019).
Kaur, H., Bose, C. & Mande, S. S. Tryptophan metabolism by gut microbiome and gut-brain-axis: an in silico analysis. Front. Neurosci. 13, 1365 (2019).
Bender, M. J. et al. Dietary tryptophan metabolite released by intratumoral Lactobacillus reuteri facilitates immune checkpoint inhibitor treatment. Cell 186, 1846–1862.e26 (2023).
Laurans, L. et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat. Med. 24, 1113–1120 (2018).
Goubet, A.-G. et al. Prolonged SARS-CoV-2 RNA virus shedding and lymphopenia are hallmarks of COVID-19 in cancer patients with poor prognosis. Cell Death Differ. 28, 3297–3315 (2021).
Danlos, F.-X. et al. Metabolomic analyses of COVID-19 patients unravel stage-dependent and prognostic biomarkers. Cell Death Dis. 12, 258 (2021).
Peyraud, F. et al. Circulating L-arginine predicts the survival of cancer patients treated with immune checkpoint inhibitors. Ann. Oncol. 33, 1041–1051 (2022).
Geiger, R. et al. L-Arginine modulates T cell metabolism and enhances survival and anti-tumor activity. Cell 167, 829–842.e13 (2016).
Canale, F. P. et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 598, 662–666 (2021).
Messaoudene, M. et al. A natural polyphenol exerts antitumor activity and circumvents anti-PD-1 resistance through effects on the gut microbiota. Cancer Discov. 12, 1070–1087 (2022).
Grajeda-Iglesias, C. et al. Oral administration of Akkermansia muciniphila elevates systemic antiaging and anticancer metabolites. Aging 13, 6375–6405 (2021).
Montégut, L., de Cabo, R., Zitvogel, L. & Kroemer, G. Science-driven nutritional interventions for the prevention and treatment of cancer. Cancer Discov. https://doi.org/10.1158/2159-8290.CD-22-0504 (2022).
Pietrocola, F. et al. Caloric restriction mimetics enhance anticancer immunosurveillance. Cancer Cell 30, 147–160 (2016).
Bourgin, M. et al. Circulating acetylated polyamines correlate with Covid-19 severity in cancer patients. Aging 13, 20860–20885 (2021).
Mager, L. F. et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489 (2020).
Nie, X. et al. Serum metabolite biomarkers predictive of response to PD-1 blockade therapy in non-small cell lung cancer. Front. Mol. Biosci. 8, 678753 (2021).
Wu, K. et al. The gut microbial metabolite trimethylamine N-oxide aggravates GVHD by inducing M1 macrophage polarization in mice. Blood 136, 501–515 (2020).
Mirji, G. et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 7, eabn0704 (2022).
Narunsky-Haziza, L. et al. Pan-cancer analyses reveal cancer-type-specific fungal ecologies and bacteriome interactions. Cell 185, 3789–3806.e17 (2022).
Poore, G. D. et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 579, 567–574 (2020).
Stein-Thoeringer, C. K. et al. A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916 (2023).
Mok, T. et al. Phase 3 KEYNOTE-042 trial of pembrolizumab (MK-3475) versus platinum doublet chemotherapy in treatment-naive patients (pts) with PD-L1–positive advanced non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 33 (Suppl. 15), TPS8105 (2015).
Center for Devices and Radiological Health. List of cleared or approved companion diagnostic devices (in vitro and imaging tools) (FDA, 2023).
Marabelle, A. et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J. Clin. Oncol. 38, 1–10 (2020).
Snyder, A. et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 371, 2189–2199 (2014).
Rizvi, H. et al. Molecular determinants of response to anti-programmed cell death (PD)−1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 36, 633–641 (2018).
Rousseau, B. et al. The spectrum of benefit from checkpoint blockade in hypermutated tumors. N. Engl. J. Med. 384, 1168–1170 (2021).
Hellmann, M. D. et al. Nivolumab plus Ipilimumab in lung cancer with a high tumor mutational burden. N. Engl. J. Med. 378, 2093–2104 (2018).
Mezquita, L. et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 4, 351–357 (2018).
Yoon, H.-Y. et al. Association between neutrophil-to-lymphocyte ratio and gut microbiota in a large population: a retrospective cross-sectional study. Sci. Rep. 8, 16031 (2018).
Schalper, K. A. et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat. Med. 26, 688–692 (2020).
Yuen, K. C. et al. High systemic and tumor-associated IL-8 correlates with reduced clinical benefit of PD-L1 blockade. Nat. Med. 26, 693–698 (2020).
Tintelnot, J. et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 615, 168–174 (2023).
Hezaveh, K. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e8 (2022).
Moor, K. et al. Analysis of bacterial-surface-specific antibodies in body fluids using bacterial flow cytometry. Nat. Protoc. 11, 1531–1553 (2016).
Kalaora, S. et al. Identification of bacteria-derived HLA-bound peptides in melanoma. Nature 592, 138–143 (2021).
Mizukoshi, E. et al. Peptide vaccine-treated, long-term surviving cancer patients harbor self-renewing tumor-specific CD8+ T cells. Nat. Commun. 13, 3123 (2022).
Liang, J. et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundam. Res. 2, 23–36 (2022).
Cheng, K. et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via plug-and-display technology. Nat. Commun. 12, 2041 (2021).
Li, C. et al. Deep insights into the gut microbial community of extreme longevity in south Chinese centenarians by ultra-deep metagenomics and large-scale culturomics. NPJ Biofilms Microbiomes 8, 28 (2022).
Rampelli, S. et al. Shotgun metagenomics of gut microbiota in humans with up to extreme longevity and the increasing role of xenobiotic degradation. mSystems 5, e00124-20 (2020).
Wang, J. et al. The landscape in the gut microbiome of long-lived families reveals new insights on longevity and aging – relevant neural and immune function. Gut Microbes 14, 2107288 (2022).
Luan, Z. et al. Metagenomics study reveals changes in gut microbiota in centenarians: a cohort study of Hainan centenarians. Front. Microbiol. 11, 1474 (2020).
Zhang, X. et al. Sex- and age-related trajectories of the adult human gut microbiota shared across populations of different ethnicities. Nat. Aging 1, 87–100 (2021).
Liu, R. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868 (2017).
Wang, Q. et al. A metagenome-wide association study of gut microbiota in asthma in UK adults. BMC Microbiol. 18, 114 (2018).
Cui, X. et al. Metagenomic and metabolomic analyses unveil dysbiosis of gut microbiota in chronic heart failure patients. Sci. Rep. 8, 635 (2018).
Crovesy, L., Masterson, D. & Rosado, E. L. Profile of the gut microbiota of adults with obesity: a systematic review. Eur. J. Clin. Nutr. 74, 1251–1262 (2020).
Bowerman, K. L. et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat. Commun. 11, 5886 (2020).
Calderón-Pérez, L. et al. Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study. Sci. Rep. 10, 6436 (2020).
Ni, Y. et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 15, 3207–3220 (2021).
Jiao, N. et al. Alterations in bile acid metabolizing gut microbiota and specific bile acid genes as a precision medicine to subclassify NAFLD. Physiol. Genom. 53, 336–348 (2021).
Behary, J. et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 12, 187 (2021).
Solé, C. et al. Alterations in gut microbiome in cirrhosis as assessed by quantitative metagenomics: relationship with acute-on-chronic liver failure and prognosis. Gastroenterology 160, 206–218.e13 (2021).
Nakai, M. et al. Essential hypertension is associated with changes in gut microbial metabolic pathways: a multisite analysis of ambulatory blood pressure. Hypertension 78, 804–815 (2021).
Aasmets, O., Krigul, K. L., Lüll, K., Metspalu, A. & Org, E. Gut metagenome associations with extensive digital health data in a volunteer-based Estonian microbiome cohort. Nat. Commun. 13, 869 (2022).
Clooney, A. G. et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment. Pharmacol. Ther. 43, 974–984 (2016).
Imhann, F. et al. Proton pump inhibitors affect the gut microbiome. Gut 65, 740–748 (2016).
Lin, Y.-T. et al. Anti-acid drug treatment induces changes in the gut microbiome composition of hemodialysis patients. Microorganisms 9, 286 (2021).
Palleja, A. et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 3, 1255–1265 (2018).
Parker, E. P. K. et al. Changes in the intestinal microbiota following the administration of azithromycin in a randomised placebo-controlled trial among infants in south India. Sci. Rep. 7, 9168 (2017).
Singh, G. et al. The effect of gastric acid suppression on probiotic colonization in a double blinded randomized clinical trial. Clin. Nutr. ESPEN 47, 70–77 (2022).
Vich Vila, A. et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 11, 362 (2020).
Li, J. K. M. et al. A cross-sectional study on gut microbiota in prostate cancer patients with prostatectomy or androgen deprivation therapy. Prostate Cancer Prostatic Dis. 24, 1063–1072 (2021).
Thompson, N. A. et al. The MITRE trial protocol: a study to evaluate the microbiome as a biomarker of efficacy and toxicity in cancer patients receiving immune checkpoint inhibitor therapy. BMC Cancer 22, 99 (2022).
Shoji, F. et al. Chronological analysis of the gut microbiome for efficacy of atezolizumab-based immunotherapy in non-small cell lung cancer: protocol for a multicenter prospective observational study. Thorac. Cancer 13, 2829–2833 (2022).
Acknowledgements
M.F. has received funding from the Seerave Foundation. B.R. has received support from the Canadian Institute for Health Research (CIHR), Fonds de la Recherche Québec-Santé (FRQS), the Seerave Foundation, the Terry Fox Marathon of Hope Program and the Weston Foundation. J.A.W. receives support from the American Association for Cancer Research Stand Up To Cancer (SU2C-AACR-IRG-19–17), MD Anderson Cancer Center’s Melanoma Moon Shots Program, Melanoma Research Alliance (4022024) and the National Institutes of Health (NIH) (1 R01 CA219896–01A1). N.S. has received support from the European Union Horizon 2020 programme (ONCOBIOME-825410 project, MASTER-818368 project and IHMCSA-964590), European Research Council (ERC-STG project MetaPG-716575 and ERC-CoG microTOUCH-101045015), the National Cancer Institute of the NIH (1U01CA230551) and Premio Internazionale Lombardia e Ricerca 2019. L.Z. has received support from ANR grant–French-German Ileobiome 19-CE15-0029-01, European Union Horizon 2020 research and innovation programme under grant agreement number 825410 (project acronym ONCOBIOME, project title Gut OncoMicrobiome Signatures (GOMS) associated with cancer incidence, prognosis, and prediction of treatment response), European Union Horizon Europe research and innovation programme under grant agreement number 101095604–GAP-101095604 (project acronym PREVALUNG-EU, project title Personalized lung cancer risk assessment leading to stratified Interception), RHU5 “ANR-21-RHUS-0017” IMMUNOLIFE, SIGN’IT ARC foundation and SIRIC Stratified Oncology Cell DNA Repair and Tumour Immune Elimination (SOCRATE). L.Z. and G.K. have received donations from Elior and the Seerave Foundation; and.support from ANR projets blancs, Badinter Philantropia, Cancéopole Ile-de-France; Dassault, FHU CARE, Fondation pour la Recherche Médicale (FRM), Inserm (HTE), Institut National du Cancer (INCa), Institut Universitaire de France, LabEx Immuno-Oncology and Ligue contre le Cancer (Equipe labelisée).
Author information
Authors and Affiliations
Contributions
L.Z. wrote the main text except the description of the CRC and melanoma GOMS performed by A.M.T. and N.S. A.M.T. and N.S. performed the meta-analysis and mega-analysis. G.K. and J.A.W. language-edited the manuscript. M.F. and B.R. prepared the display items for the submitted manuscript.
Corresponding author
Ethics declarations
Competing interests
A.M.T. is a new employee of Microbiotica. B.R. has received grants from AstraZeneca, Bristol Myers Squibb, Davoltera, Kaleido, Merck and Vedanta outside the submitted work; and has a patent pending (G17004–00006-AD, use of castalagin or analogues thereof for anticancer efficacy and to increase the response to immune-checkpoint inhibitors). G.K. has held research contracts with Daiichi Sankyo, Elior, Kaleido, Lytix Pharma, PharmaMar, Samsara, Sanofi, Tollys, Vascage and Vasculox/Tioma; is on the Board of Directors of the Bristol Myers Squibb Foundation in France; is a scientific co-founder of EverImmune, Osasona Therapeutics, Samsara Therapeutics and Therafast Bio; and is an inventor in patents covering therapeutic targeting of ageing, cancer, cystic fibrosis and metabolic disorders. J.A.W. is an inventor on a US patent application (PCT/US17/53.717) submitted by the University of Texas MD Anderson Cancer Center, which covers methods to enhance immune-checkpoint inhibitor responses by modulating the microbiome; reports compensation for speaker’s bureau and honoraria from Bristol Myers Squibb, Dava Oncology, Exelixis, Gilead, Illumina, Imedex, MedImmune, Omniprex, PeerView and Physician Education Resource; has served as a consultant or advisory board member for AstraZeneca, Bristol Myers Squibb, EverImmune, GlaxoSmithKline, Merck Novartis, Roche/Genentech, Micronoma and OSE therapeutics; and receives stock options from Micronoma and OSE therapeutics. L.Z. is the scientific cofounder and President of the scientific advisory board of EverImmune, a company devoted to the use of commensal microorganisms (Oncobax) for the treatment of cancers; has received research grants from 9 meters, Daichi Sankyo, EverImmune and Pileje; is a former member of the board of directors of Transgene; and is a former consulting expert for Bristol Myers Squibb, GlaxoSmithKline, Lytix biotherapeutics and Tusk. M.F. and N.S. declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks G. Trinchieri, M. van den Brink and J. Yu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov/
Supplementary information
Glossary
- 16S ribosomal RNA sequencing
-
Targeted, high-throughput method that consists of amplifying and sequencing the small-subunit ribosomal 16S RNA (rRNA) gene present in a sample (for example, a microbial community in stools). Sequences are generally the result of targeted amplification of one or more variable regions within the 16S rRNA gene, and can be used to profile the taxonomic composition of both archaeal and eubacterial members of the microbial community. This method provides lower taxonomic resolution than shotgun metagenomics but is generally less computationally intensive. Nevertheless, a variety of processing steps can introduce bias and limit the ability to combine data from different studies.
- Alpha and beta diversities
-
Alpha diversity or richness measures the variability of species within a faecal sample while beta diversity accounts for the differences in composition between individuals.
- Dysbiosis
-
Imbalance of microbiota that involves changes in composition and/or functions.
- Exfoliome
-
Shed intestinal luminal cells contained within faecal samples.
- Exposome
-
Encompasses the large number of individual exposures from various origins, such as chemical, physical, biological or psychological stimuli, and also takes into account the time dimension of the exposure (short or long, early or late, punctual or repeated). The exposome has an impact on individual physiology and, to a larger extent, overall health.
- Leave-one-dataset-out
-
Analytical approach in which data from one cohort are set aside as an external validation set whereas data from the remaining cohorts are pooled together as a single training set, iterating along all the cohorts.
- Linear discriminant analysis effect size
-
Determines the features (organisms, clades, operational taxonomic units, genes or functions) most likely to explain differences between classes by coupling standard tests for statistical significance with additional tests encoding biological consistency and effect relevance.
- Mega-analysis
-
Analysis that gathers raw data across multiple studies.
- Random forest model
-
Supervised machine learning algorithm used to solve classification and regression problems that averages multiple decision trees.
- Short-chain fatty acids
-
Metabolic by-products derived from the fermentation of carbohydrates by anaerobic bacteria in the gut.
- Shotgun metagenomic sequencing
-
Non-targeted, high-throughput method that consists of sequencing all DNA present in a sample (for example, a microbial community). These DNA sequences are computationally analysed and can be used to profile the taxonomic composition of members of the microbial community, including bacteria, fungi and viruses.
- Species-level genome bins
-
Large-scale metagenomic analyses have uncovered a myriad of bacteria never before described. The species-level genome bin (SGB) nomenclature enables the annotation of bacterial sequences to the species level.
- Stress ileopathy
-
Ileal mucosa atrophy associated with dominance of sympathetic over cholinergic signalling provoked by intra-intestinal and extra-intestinal malignancies or chronic inflammatory disorders (inflammatory bowel disease, stroke).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, A.M., Fidelle, M., Routy, B. et al. Gut OncoMicrobiome Signatures (GOMS) as next-generation biomarkers for cancer immunotherapy. Nat Rev Clin Oncol 20, 583–603 (2023). https://doi.org/10.1038/s41571-023-00785-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00785-8
This article is cited by
-
A gut microbial signature for combination immune checkpoint blockade across cancer types
Nature Medicine (2024)
-
A microbial iron fist to fight tumors
Nature Immunology (2024)
-
Longitudinal gut microbiome changes in immune checkpoint blockade-treated advanced melanoma
Nature Medicine (2024)
-
Role of the microbiota in response to and recovery from cancer therapy
Nature Reviews Immunology (2023)