Abstract
Given that cancer mortality is usually a result of late diagnosis, efforts in the field of early detection are paramount to reducing cancer-related deaths and improving patient outcomes. Increasing evidence indicates that metastasis is an early event in patients with aggressive cancers, often occurring even before primary lesions are clinically detectable. Metastases are usually formed from cancer cells that spread to distant non-malignant tissues via the blood circulation, termed circulating tumour cells (CTCs). CTCs have been detected in patients with early stage cancers and, owing to their association with metastasis, might indicate the presence of aggressive disease, thus providing a possible means to expedite diagnosis and treatment initiation for such patients while avoiding overdiagnosis and overtreatment of those with slow-growing, indolent tumours. The utility of CTCs as an early diagnostic tool has been investigated, although further improvements in the efficiency of CTC detection are required. In this Perspective, we discuss the clinical significance of early haematogenous dissemination of cancer cells, the potential of CTCs to facilitate early detection of clinically relevant cancers, and the technological advances that might improve CTC capture and, thus, diagnostic performance in this setting.
Similar content being viewed by others
Introduction
Globally, cancer is the second most common cause of death1, driven by mortality rates that increase with disease stage at diagnosis2. Detecting cancer at an advanced stage severely limits treatment options and typically results in a poor prognosis. Indeed, the options available for patients with metastatic solid tumours are very rarely curative. Therefore, efforts in the field of early cancer detection and diagnosis are paramount to improving patient outcomes. Many strategic programmes focused on early cancer detection have been launched worldwide, including Europe’s Beating Cancer Plan by the European Commission, the UK Research and Innovation Accelerating detection of disease challenge, and the US NIH/National Cancer Institute Cancer Moonshot initiative.
In the UK, advances in early cancer diagnosis over the past 5 years include the implementation of Rapid Diagnostic Clinics (RDCs), which have resulted in the detection of malignancies in 7% of patients referred owing to non-site-specific symptoms in England who would otherwise have experienced substantial delays in diagnosis3. The Welsh RDCs have reduced the mean time to a definitive diagnosis from 84.2 days to as low as 5.9 days (or 40.8 days if further investigations were required) for patients with vague symptoms raising suspicion of cancer4. These RDCs not only decrease the time to diagnosis for many patients but potentially also offer an ideal setting for the testing and application of novel diagnostic biomarkers of cancer. Of note, however, the COVID-19 pandemic has caused delays in cancer diagnosis for many patients, subsequently increasing morbidity and mortality5,6. Better biomarkers and novel approaches that facilitate cancer detection are needed to accelerate diagnosis and mitigate the delays and challenges presented by pandemics such as those caused by COVID-19.
The current gold standard for cancer diagnosis is histopathology, which usually involves an invasive procedure to biopsy solid tumour tissue. Indeed, multiple biopsy samples can be required, yet this approach still provides only limited information on tumour heterogeneity. In addition, biopsy samples cannot be obtained from individuals who have no clinical evidence of cancer, severely limiting the capacity for early detection, or from those who are not fit enough to undergo such invasive procedures. Notably, advanced imaging technologies, such as multi-parametric MRI and PET–CT, can only detect primary tumours and metastases that are already well established, consisting of >109 cells7. Therefore, research on liquid biopsy assays has expanded rapidly, with the exploration of many different biomarkers in distinct bodily fluids for the evaluation of various solid malignancies8. Liquid biopsy sampling is minimally invasive or non-invasive, enabling repeat sampling in the same individual to detect cancer as well as to assess treatment response and/or monitor for disease progression9. Furthermore, circulating cancer-derived material can originate from both primary and (micro)metastatic sites, potentially providing a better representation of the entire heterogeneous tumour cell population than that afforded by tissue biopsy sampling10.
Metastasis is the main cause of cancer-related death and can occur at an early stage of tumour development in patients with aggressive cancers11. The first step of metastatic dissemination involves cancer cell invasion into the blood circulation via which the cells can spread to other parts of the body. Studies focused on disseminated tumour cells (DTCs) in the bone marrow of patients with breast cancer have revealed early metastatic spread to distant sites even in patients with small, early stage tumours12. However, DTCs can enter dormancy such that the metastatic lesion might form and subsequently be detected many years after initial cancer cell dissemination13,14. Therefore, circulating tumour cells (CTCs) in the blood might be the first indicators of the early steps of cancer metastasis, might enable monitoring for this process in a minimally invasive manner and have the potential to be applied as a tool for the early detection of aggressive cancers. CTCs can have prognostic utility as evidenced by the FDA approvals of the CELLSEARCH platform for the prediction of progression-free and overall survival in patients with metastatic prostate cancer15, breast cancer16,17 or colorectal cancer (CRC)18,19. The prognostic value of CTCs has also been demonstrated in patients with bladder cancer20,21, head and neck cancer22,23, and pancreatic cancer24. However, the research community is increasingly focusing on the potential use of CTCs in early cancer detection and diagnosis. Given that not all cancers are lethal and some remain latent for many years, the diagnosis of slow-growing cancers can lead to overdiagnosis and overtreatment, exposing patients to more harm than potential benefits and increasing the costs incurred by health-care systems for unnecessary diagnostic procedures and treatments25. Therefore, for liquid biopsy assays to be effective tools for early cancer diagnosis, they must focus on identifying aggressive cancers that require immediate treatment, and the analysis of captured CTCs might have an advantage in this regard.
We hypothesize that aggressive cancers with metastatic potential will release CTCs very early during tumorigenesis. Herein, we discuss the literature on the metastatic process and CTC analysis with a particular emphasis on their salience at early stages of cancer development, highlighting research advances to support our hypothesis. We also provide our thoughts on future directions for research to facilitate the application of CTCs in early cancer detection, particularly technological improvements to increase the sensitivity of CTC assays. Although CTCs might also be used to detect minimal residual disease, this application is beyond the scope of this Perspective and has been reviewed elsewhere26.
Cancer metastasis
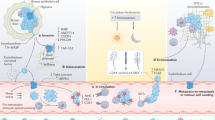
Metastatic colonization of secondary sites is initiated by cancer cells accumulating alterations affecting genes involved in various processes identified as the hallmarks of cancer27,28, which support the migration, invasion, survival and eventual outgrowth of these cells beyond their tissue of origin. The metastatic cascade starts with local invasion of the primary tumour cells into their surrounding microenvironment and subsequent migration across the endothelial barrier, intravasating into the blood or lymphatic system29. After entering the circulation, CTCs are carried in the blood to other body sites, where they can extravasate, proliferate and establish metastatic lesions (Fig. 1).
Dissemination of cancer cells from the primary tumour into circulation (step 1) can involve either single cells or cell clusters containing multiple circulating tumour cells (CTCs) as well as immune cells and platelets, known as microemboli. CTCs that can survive in circulation (step 2) can exit the bloodstream (step 3) and establish metastatic tumours (step 4), or they can enter dormancy and reside in distant organs such as the bone marrow (inset). Dormant disseminated cancer cells can regain proliferative capacity at a later stage and establish overt metastatic lesions after a long latency of several months or even years, depending on the primary tumour type.
Cancer cells can undergo epithelial-to-mesenchymal transition (EMT) to facilitate their detachment from the primary tumour and intravasation into the blood circulation30. EMT involves the loss of epithelial characteristics, for example, downregulation of the adhesion molecule E-cadherin, and the acquisition of mesenchymal characteristics, including expression of the cytoskeletal protein vimentin31. Vimentin has been found to be overexpressed in numerous malignancies, such as breast cancer32,33 and extrahepatic cholangiocarcinoma34, and is associated with cancer cell invasiveness and metastasis35. The process of EMT is mediated through TGFβ, Wnt and Notch signalling and can be controlled by the transcription factors SNAI1 (also known as SNAIL), SNAI2 (SLUG), TWIST1 and FOXC2 (ref. 36). This invasion process can involve single cancer cells or clusters of tumour cells. Intravasation of single cells can be mediated through TGFβ signalling37, whereas the intravasation of cell clusters might be initiated by primary tumour hypoxia38, although various alternative pathways probably also contribute to this process.
One of the most crucial and under-investigated steps in the metastasis process involves CTC intravasation and survival in the blood39 prior to their extravasation at a secondary site. After infiltrating the circulation, CTCs are subject to a harsh environment in which they must undergo complex, adaptive processes in order to survive. For example, both hydrodynamic forces40 and the actions of immune cells can result in CTC death41. Nascent CTCs can also undergo anoikis after detachment from the extracellular matrix42, and sudden changes in cellular oxygen levels after entering the blood can require rapid adaptation of cancer cells43.
Extravasation of CTCs involves their interaction with endothelial cells lining the surrounding blood vessel, a process mediated through integrin adhesion44. Provided that the new niche has a suitable microenvironment for cancer cell survival and growth, the invading CTCs can proliferate and establish overt metastatic lesions (Fig. 1). Therefore, only if the metastatic cascade is successfully completed will patients develop secondary tumours, at which point their treatment options will be limited. By contrast, detection of CTCs in patients with early stage cancers might not necessarily preclude curative therapy as long as they have no signs of overt metastases. However, CTC detection might indicate the presence of an aggressive cancer with high propensity for metastatic dissemination, warranting the addition or intensification of adjuvant systemic therapy with the aim of eradicating any occult micrometastases. Conversely, an absence of CTCs might provide opportunities for treatment de-escalation in some disease settings provided that the detection methods have sufficient sensitivity.
Early dissemination of cancer cells
Cancer cell spread occurs early but is usually detected late
Metastases are usually detected at late stages of cancer development when cancer cells have evolved to form a large tumour burden beyond the primary organ of origin, which typically precludes curative therapy and results in a short survival duration. Nevertheless, many patients with cancer do not die from metastatic disease until years after their initial diagnosis, with several lines of evidence indicating that cancer cell dissemination can be an early event in tumorigenesis. In early work from the 1950s onwards, calculations based on cell proliferation rates suggested that primary tumours as small as 5 mm in diameter can metastasize to multiple sites months or even years before their detection45,46,47. Subsequently, the detection of DTCs has provided direct evidence that the initial dissemination to distant sites often occurs at an early stage of cancer development. Indeed, DTCs have been identified in patients with various early stage cancers48,49, including the earliest stages of gastric cancer48, invasive breast cancer12 and even ductal carcinoma in situ50,51,52. Notably, the detection of DTCs in the bone marrow of patients with CRC, a cancer type in which overt skeletal metastases are very rare, suggests that cancer cell spread and outgrowth can be two distinct biological processes53. Patients with breast ductal carcinoma in situ can also have clinically undetectable micrometastases or occult secondary lesions52. This phenomenon is perhaps attributable to micro-invasion of tumours (with no invasive foci >1 mm), which are only detectable by immunocytochemistry of bone marrow aspirates52. Furthermore, CTCs have been detected in blood samples years before a clinical cancer diagnosis54. In mouse models, DTCs were detectable soon after orthotopic implantation of a small number of breast cancer cells50, and CTCs with mesenchymal features were detected in the blood prior to pancreatic tumour detection by rigorous histological analysis55. These findings emphasize that EMT and dissemination of cancer cells can precede tumour discovery.
Studies using next-generation sequencing technologies to measure the genomic divergence between primary and metastatic tumours have suggested that early micrometastasis, before primary tumour detection, is a common feature of many human cancers56,57,58. For example, exome-sequencing data revealed a low level of genomic divergence between paired primary colorectal tumours and brain or liver metastases57. In addition, the fact that cancers of unknown primary account for 3–5% of all malignancies59 provides further hints of early metastatic dissemination while the primary tumour remains undetectable with current diagnostic technologies. Furthermore, for more than 50 years it has been known that cancer can sometimes be inadvertently transmitted to patients transplanted with apparently healthy organs, suggesting that cancer cell dissemination occurred at an early stage in tumour development and remained undetected in the donor organs49.
Cancer cell dormancy can explain the late detection of metastasis
Dormancy of DTCs might be the main reason for the late detection of metastasis, long after cancer cell dissemination early in primary tumour development. DTC dormancy, during which the cells usually have a low proliferative index (as assessed by Ki67 staining)53, is described as a state of G0–G1 phase mitotic arrest from which cells have the capacity to regain proliferative traits and establish overt metastatic lesions, often many years later13. Exit from the dormant phase can occur even after surgical removal of the primary tumour60. For example, ~40% of patients with prostate cancer treated by radical prostatectomy have biochemical recurrence, suggesting the presence of DTCs or undetectable micrometastases at the time of surgery61,62. DTCs detected many years after primary tumour resection must have been released into circulation before the apparently successful surgical treatment, forming occult micrometastases at a later stage14.
In various mouse models, DTCs that are released early in tumorigenesis can exhibit metastatic capacity63,64 although they often initially enter dormancy65. DTCs in a state of cell cycle arrest are hypothesized to be insusceptible to therapies that target the increased proliferative activity of tumour cells66,67. DTCs obtained from the bone marrow of patients with breast and gastrointestinal cancers have been shown to have a very low Ki67 index53; therefore, these non-cycling cells are likely to be more resistant to cytotoxic therapies such as the typical chemotherapy regimens administered to patients with solid tumours. However, therapeutic agents with activity against DTCs and CTCs that are in G0–G1 phase of the cell cycle (for example, immune-checkpoint inhibitors such as anti-PD-(L)1 antibodies)68 or senolytic drugs (for example, BCL-2 inhibitors) that can re-activate the apoptotic pathway to eliminate cells under cell cycle arrest might be effective in eradicating micrometastases69. In addition to the current limitations in detecting micrometastases, DTC dormancy leads to understaging, and thus undertreatment, of many aggressive tumours, presenting an obstacle to effective eradication of cancer and leading to later metastatic relapse. However, cancer cell dormancy might also provide a therapeutic window of opportunity to cure cancer before the metastases are well established with many diverse subclones and a protective local ecosystem. Nevertheless, research into cancer cell dormancy is limited owing to the invasive and technically challenging nature of sample acquisition, particularly as DTCs are most commonly obtained from bone marrow70. Further understanding of the mechanisms underpinning cancer cell dormancy is crucial to predict metastatic potential, and Cancer Research UK and the US National Cancer Institute have jointly proposed a Cancer Grand Challenge to investigate this major issue. Currently, biomarkers for measuring local cancer cell invasion are lacking; however, once invasion into circulation has taken place, CTCs might hold a wealth of information for monitoring micrometastasis and assessing the risk of eventual overt metastasis.
Circulating tumour cells
CTCs are cancer cells that have shed from the solid tumour and entered the circulation. As previously mentioned, they have the capacity to extravasate at a different anatomical site and establish overt metastatic lesions. Although their existence has been known for more than 150 years71, only in the past few decades has technology advanced to enable experimental investigation of CTCs and evaluation of their biomarker utility72. Anti-epithelial cell adhesion molecule (anti-EpCAM) antibody-based CTC capture technologies have played an important part in the initial clinical application of CTCs as a biomarker and one of these systems, the CELLSEARCH platform, was the first CTC detection technology approved by the FDA for clinical use73. However, the EMT phenomenon is important to consider when isolating CTCs given that expression of EpCAM is typically reduced during this transition74. Epitope-independent isolation strategies might therefore be more effective in isolating all subtypes of CTCs. For example, CTCs with both epithelial and mesenchymal characteristics have been identified in patients with prostate cancer using the cell size-based Parsortix isolation system75,76 (Fig. 2). Other CTC isolation methods based on cell size or density, including the Isolation by Size of Epithelial Tumor cells (ISET)77, FaCTChecker78 and Oncoquick79 platforms, also have demonstrated utility for harvesting CTCs independently of epithelial marker expression. Evidence indicates that certain subtypes of CTCs (such as those with partial EMT or mesenchymal phenotypes) have greater potential to seed distant metastases and are associated with a poor prognosis in patients with various cancers76,80. In addition, emerging evidence suggests that not only are CTC numbers increased during sleep or rest phases but these CTCs also have an increased ability to metastasize than those generated during active phases81.
The immunofluorescent microscopy images illustrate three subsets of circulating tumour cells (CTCs) with differential expression of characteristic markers isolated from patients with prostate cancer using the cell size-based Parsortix isolation system. The first row shows a representative epithelial CTC, which is positive for the epithelial marker cytokeratin (CK) and negative for the mesenchymal marker vimentin (VIM) and the leukocyte marker CD45. The middle row depicts two CTCs undergoing epithelial-to-mesenchymal transition as determined by positive staining for both CK and VIM. The last row features two mesenchymal CTCs, which are positive for VIM and negative for CK. Reprinted with permission from ref. 76, American Association for Cancer Research.
Clusters of CTCs with and without leukocytes might have an important role in seeding distant metastases82. These CTC clusters (microemboli) are defined as groups of two or more CTCs and can consist of CTCs alone (homotypic) or can include various stromal cells, such as cancer-associated fibroblasts, and/or platelets and immune cells (heterotypic)83,84. The greater metastatic capacity of CTC clusters compared with individual CTCs has been demonstrated in mouse models of breast cancer85, with one study finding that 97% of metastases originated from CTC clusters86. Data indicates that CTC clustering can lead to DNA hypomethylation of binding sites for transcription factors that promote cell stemness and proliferation, thus enhancing their metastatic potential87. In addition, a study involving patients with breast cancer found that neutrophils interacting with CTCs induced upregulation of genes involved in cell cycle progression in cancer cells, perhaps leading to more efficient formation of metastases82. Similar findings have been reported for platelet-associated CTCs88. Furthermore, non-cancer cells present in such heterotypic clusters might protect the CTCs from hydrodynamic shear stress and immune attack89. Given that CTCs in clusters still also engage in some degree of cell–cell adhesion, they can provide stimuli to one another during their circulation in the blood, potentially protecting each other from anoikis83. Clustering of CTCs (which might each have different phenotypes) is also likely to better enable them to foster a supportive ecosystem after extravasation87. Data from mouse models suggest that, owing to their larger size, CTC clusters are more likely than single CTCs to become mechanically trapped in capillaries, increasing their potential to extravasate at secondary sites90. This phenomenon has also been noted in patients with metastatic breast and cervical cancer, with CTC retention observed in the lung microvasculature91. CTC clusters have also been reported in patients with prostate cancer but at a varying rate depending on the isolation strategy applied92,93,94. These findings suggest that CTC clusters are associated with metastasis development, and this has been confirmed in a cohort of patients with breast cancer81. Importantly, CTC clusters might provide clinically relevant information on tumour heterogeneity. For example, one study involving patients with prostate cancer reported varying levels of cytokeratin expression among CTCs within the same cluster95, and evidence derived from patients with breast cancer indicates that expression of specific types of cytokeratins (such as cytokeratin 16) might affect the biology and metastatic potential of CTCs96.
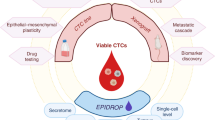
A multitude of characteristics can be measured in CTCs, including genetics and epigenetics as well as protein levels, which might help us to understand many processes involved in the formation of metastases (Fig. 3). For example, single-cell whole-exome sequencing of CTCs derived from 10 patients with localized high-risk prostate cancer revealed thousands of single-nucleotide variants, insertions and/or deletions (indels), and copy-number alterations, which were ultimately associated with pathways involved in telomere preservation, DNA damage repair and response to docetaxel chemotherapy97. Genetic analysis of CTCs might also provide additional information on tumour mutational burden and intrapatient heterogeneity in mutational profiles98. Furthermore, evidence indicates that epigenetic profiling of CTCs might be clinically important, with SOX17 hypermethylation noted in CTCs from a substantial proportion of patients with breast cancer, including up to 54% of those with early stage disease99, suggesting silencing of this tumour suppressor gene. Increased hypermethylation of the tumour suppressor genes CST6 and BRMS1 has also been noted in CTCs from patients with metastatic breast cancer compared with CTCs derived from those with apparently localized disease100. Hence, CTC analysis might help us to better understand EMT and intravasation mechanisms as well as the control of DTC dormancy, and also help us to discriminate between latent and aggressive cancers.
Multi-molecular analyses of circulating tumour cells (CTCs) can provide a wealth of information on various processes involved in cancer dissemination and metastasis, including cancer cell intravasation, extravasation, dormancy and epithelial-to-mesenchymal transition (EMT). In addition, successful detection of cancer cell dissemination in the form of CTCs can provide less invasive biomarkers for cancer diagnosis and prognostication — considering that the presence of these cells in the blood inherently differentiates clinically relevant aggressive cancers from indolent tumours — as well as for predicting and monitoring treatment response.
In addition, CTC quantification might be indicative of tumour burden in patients with aggressive cancers as reported in a study involving patients with primary lung adenocarcinoma101. However, in malignancies such as prostate cancer, for which the cancer grade group is determined by pathological grade rather than tumour size (T stage), CTC positivity and number are more dependent on tumour aggressiveness than purely on tumour burden102. Interestingly, CTCs have been found to pass through the blood–brain barrier and can therefore be detected in patients with primary brain tumours103. Although most of the published research on CTCs to date has been focused on their prognostic capacity in patients with advanced-stage cancers, exploration of the utility of CTCs in the field of early cancer detection is now increasing (Fig. 3).
CTCs in early detection of cancer
Early research on CTCs did not explore their utility in the diagnosis of early stage cancers because CTCs were initially thought to be a feature of advanced-stage disease and also owing to the limitations of technologies to detect such scarce cells104. However, evidence suggests that the process of local invasion and intravasation of cancer cells can occur quickly, in a timescale of hours105, and therefore CTC detection might be able to precede a clinical cancer diagnosis. This possibility is supported by data from genetically engineered PLCY mouse models of pancreatic intraepithelial neoplasia and pancreatic ductal adenocarcinoma, in which fluorescently tagged transgenic pancreatic epithelial cells were detectable in the circulation and seeded the liver before frank pancreatic tumours could be detected by histopathological and fluorescence imaging55.
A number of clinical studies have assessed the potential of CTCs for cancer detection using blood samples from patients with a known cancer diagnosis (Table 1). For example, CTCs have been detected in patients with early stage (stage I–IIIA) breast cancer16,17,106,107, with more than one CTC detected in 20% of patients with stage I disease, 26.8% with stage II disease and 26.7% with stage III disease108. CTCs have also been detected in patients with non-metastatic CRC, including stage I and II disease, using the CELLSEARCH technology19 and the CellMax microfluidic platform (which also involves anti-EpCAM antibody-based cell capture)109. In patients with non-metastatic prostate cancer, CTCs have also been detected using the CELLSEARCH technology110,111; using the Parsortix isolation system based on cell size and deformability, we detected CTCs in >50% of patients with localized disease76,102. In a study using size-based cell filtration followed by morphological characterization, CTCs were detected in 49% of patients with stage I non-small-cell lung cancer (NSCLC), which was not different from the frequency of detection in patients with stage II to IV disease (48%, 48% and 52%, respectively)77. Furthermore, a meta-analysis of 18 prospective studies found that CTC positivity is a promising biomarker for predicting unfavourable overall survival in patients with early stage NSCLC (HR 3.53, 95% CI 2.51−4.95; P < 0.00001)112, emphasizing the potential of CTCs to predict aggressive cancers and thus potentially guide the development of novel treatment strategies. In the context of pancreatic cancer, CTCs were detectable using the EpCAM-based NanoVelcro CTC chip in 60% of patients with stage II disease, and CTC positivity discriminated patients with pancreatic ductal adenocarcinoma from those with non-adenocarcinoma pancreatic diseases with a sensitivity of 75% and specificity of 96.3% (AUC 0.867, 95% CI 0.798–0.935; P < 0.001)113.
In a prospective study to predict the biopsy-based diagnosis of 98 individuals with suspected prostate cancer prior to biopsy, CTC detection using the Parsortix isolation system was strongly correlated with clinically significant cancer102 (Table 1). The study cohort included patients with symptoms suggestive of prostate cancer, concerning serum prostate-specific antigen (PSA) levels and/or an abnormal digital rectal examination. Clinically significant cancer was defined based on serum PSA level, Gleason score and clinical stage114,115. CTC positivity was defined as any epithelial CTC (CK+vimentin–CD45−), any ‘EMTing’ CTC (CK+/Vimentin+/CD45−) and/or more than three mesenchymal CTCs (CK−vimentin+CD45−)102. CTC positivity score (as defined above) combined with serum PSA levels predicted the biopsy-based diagnosis of clinically significant prostate cancer with an AUC of 0.869, and additional inclusion of transcriptomic analysis of CTCs using a 12-gene panel increased predictive accuracy, with an AUC of 0.927 (ref. 102). In a study involving 265 asymptomatic individuals without a cancer diagnosis but with risk factors for various malignancies (including a family history of cancer, or lifestyle factors or medication usage associated with increased risk), 132 (49.8%) had detectable CTCs using the ISET technology116. Although this high CTC positivity rate requires independent validation, follow-up tests performed within 10 months of CTC detection revealed early cancerous lesions in 20% of CTC-screened individuals; prostate-specific membrane antigen-based PET scans provided evidence of early stage prostate cancer in 50% of men with physiologically normal serum PSA levels but detectable CTCs116. In another study using the ISET technology in 168 patients with chronic obstructive pulmonary disease, the 5 patients with detectable CTCs all subsequently had NSCLC diagnosed within 1–4 years through annual CT-based screening54. Importantly, all 5 patients had resectable tumours and none had evidence of disease recurrence by CT or ISET at 12 months after surgery. In a larger screening study using the same technology in a cohort of 614 patients with chronic obstructive pulmonary disease, CTCs were identified in 5 patients found to have prevalent lung cancer, although the sensitivity was only 26.3%117. Moreover, baseline CTC status did not predict the development of 19 interval lung cancers missed on initial low-dose CT screening or extrapulmonary cancers117 (Table 1).
Together, these studies show that CTCs have the potential to be used in the early detection and/or diagnosis of clinically relevant cancers, although further investigations and technical improvements are required. Currently, many ongoing clinical trials are investigating the use of CTCs for early cancer diagnosis, including the PROLIPSY trial for prostate cancer (NCT04556916) and similar trials for breast cancer (NCT03511859), NSCLC (NCT02380196), CRC (NCT05127096) and pancreatic cancer (PANCAID). If these clinical trials produce positive results, the use of CTCs in early cancer detection might become a reality.
Future directions
Although the literature to date suggests that CTC analyses can provide viable biomarkers with applications in various aspects of malignancy (from diagnosis to monitoring treatment response), more research is required to develop CTCs as reliable biomarkers for early cancer detection. Despite the remaining challenges, emerging data underscore the promise of liquid biopsy of CTCs in early cancer detection. Most importantly, owing to their integral link with metastasis, and thus aggressive cancers, CTCs have an advantage over many other non-invasive or minimally invasive biomarkers in specifically identifying invasive cancers for early therapeutic intervention at a stage when the disease is still curable. This advantage might also help to avoid overdiagnosis of indolent cancers, which is a major issue in many malignancies such as prostate, breast, lung and thyroid cancers118. Here, we share our thoughts on the directions of future developments to accelerate the application of CTCs in early cancer detection (Box 1).
Maximizing CTC detection
Currently, the main issue restricting the use of CTCs for early cancer detection relates to the scarcity of these cells in routine blood sample volumes, which limits their sensitivity in the detection of cancer117; therefore, it is important to maximize the number of CTCs available for analysis. Given the inherently low numbers of CTCs released by early stage cancers, however, another obvious approach is to improve the sensitivity of the detection technologies. Capturing all CTCs has proven difficult with epitope-dependent isolation strategies and, subsequently, research has been focused on developing and applying isolation methods based on cell size, density or morphology to increase CTC yield54,75. Each of these technologies has its limitations, such as the omission of EpCAM-negative CTCs undergoing EMT with traditional epitope-based isolation platforms or of small CTCs with devices involving selection based on cell size. Thus, a combination of multiple selection methods might be more efficient in gathering the CTCs to study; however, although complex sequential selection strategies are likely to increase CTC purity, they might also be accompanied by an increase in cell loss during each processing step. Continued validation of CTC detection technologies will be key to ultimately obtaining approval of such systems for early cancer detection. This process might involve standardization of technologies with interlaboratory ring trials to assess reproducibility and data comparability as is being performed by the European Liquid Biopsy Society, which could eventually lead to recommendations on minimally required procedures for reporting studies based on CTCs. These recommendations will include requirements regarding pre-analytical and quality-control processes that enable optimal results with the use of CTCs as clinical biomarkers. Pre-analytical factors, such as the time and anatomical site of blood draw, needle size and type of blood collection tube, ambient temperature, and storage conditions, should all be carefully considered and outlined in CTC analysis protocols. Machine learning and other artificial intelligence approaches might be applied to large datasets in wide-scale CTC analyses, and have already been used to investigate CTCs from patients with ovarian cancer119. However, these improvements might still be insufficient to realize the potential of CTC analysis for early cancer detection; increasing the absolute number of CTCs to be isolated through blood sampling at the optimal anatomical location and time, and perhaps even increasing the sample volume, might be the key. Besides blood, shed cancer cells can be detected in other body fluids, such as lymph or cerebrospinal fluids, but their isolation requires more invasive sampling procedures.
The use of different blood vessels for sample acquisition has already been explored to increase the chance of detecting CTCs. For example, blood sampling from the pulmonary vein in patients with NSCLC120, the portal vein in those with pancreatic cancer121 or the mesenteric vein in those with CRC122 has been shown to increase the number of CTCs detected compared with peripheral blood sampling. However, these sites are difficult to sample routinely as required for application in cancer detection. As noted previously, data from a study in patients with breast cancer suggests that the time of blood sampling is an important consideration in capturing a sufficient number of CTCs for downstream analysis81. A substantially higher number of single CTCs and CTC clusters were detected in samples collected at 4:00 am (rest phase) compared to 10:00 am (active phase), with the data suggesting that 78.3% of CTCs are released during the rest phase at night81. This new information might lead to researchers altering the time of blood sampling to enhance CTC collection. Explanations for the differences in day and night time release of CTCs have been proposed123,124; further studies to determine the influence of circadian rhythms and other physiological conditions on CTC release for each cancer type should be carried out to enable the development of protocols for maximal CTC capture.
For clinical diagnostic applications, the smaller the volume of blood the better, ideally no more than the volume collected in a single 10-ml blood collection tube. The current routine blood sample volume used for CTC analysis is ≤7.5 ml (refs. 75,125). Nevertheless, the utility of CTCs for early cancer detection will be improved if the blood volume interrogated for these cells can be substantially increased. In this regard, Kim et al.126 developed a temporary indwelling intravascular aphaeretic system connected to an external anti-EpCAM antibody-based microfluidic herringbone graphene oxide CTC chip that enabled sampling of 1–2% of the whole blood volume in canine models and the isolation of injected MCF-7 breast cancer cells. Apheresis systems have similarly been used in patients with metastatic prostate or breast cancer to markedly increase CTC isolation (by up to 75-fold compared with the numbers harvested from 7.5 ml of blood) through the sampling of large volumes of blood127,128. However, this approach might be too complicated and invasive for use in a routine diagnostic test, particularly for cancer screening. A simpler device, named CellCollector, has also been developed to capture CTCs from a large volume of blood. CellCollector consists of an anti-EpCAM antibody-coated medical Seldinger guidewire that can capture CTCs in vivo following insertion into a cubital vein through a standard cannula129. This device enabled the detection of CTCs in 12 of 12 patients with breast cancer and 10 of 12 patients with NSCLC, including those with early stage, non-metastatic disease, whilst no CTCs were detected in volunteers without cancer129. Another study using CellCollector detected CTCs in 108 (58%) of 185 applications (before and after treatment) among 50 patients with lung cancer, compared with 23 (27%) of 84 applications using the CELLSEARCH platform130. However, these devices remain at an experimental stage of development and do not capture CTCs with only mesenchymal features, which might be more strongly associated with unfavourable clinical outcomes than other subtypes of CTCs76.
The development of wearable devices that can monitor a condition over time is an important direction for future research in the field of clinical diagnostics. If CTCs passing through a vein could be detected using an imaging device worn on the hand or arm, an efficient and non-invasive approach to early cancer detection could become a reality. Such technology will not only enable the identification of CTCs in a much larger blood volume but also facilitate monitoring of the temporal differences in CTC release. Development of a wearable sensor device will require the optimization of injectable probes, such as those with the capacity to generate near-infrared light, to label CTCs in vivo for detection, although in vivo CTC detection without labelling has also been reported in patients with melanoma (discussed further below)131. Clearly, the development of both CTC-specific markers and the sensor device is currently very challenging considering the potential background noise of such an approach to CTC detection in vivo. Advances of relevance for in vivo imaging of cancer include the optimization of surface-enhanced Raman scattering nanoparticles that, when coupled with spatially offset Ramen spectroscopy, could successfully image glioblastoma tumours in mice132. This study importantly demonstrates the feasibility of developing probes and coupled detection systems that can successfully penetrate tissue to the substantial depth required for diagnostic imaging132. Single-cell in vivo imaging of CTCs has already been demonstrated in mouse models of CRC using real-time confocal fluorescence microscopy133, and the technological developments and potential applications of in vivo imaging of CTCs have been reviewed elsewhere134. In mouse models of melanoma, CTCs could also be detected using melanin as an intrinsic marker coupled with in vivo photoacoustic flow cytometry135,136. Further optimization and subsequent clinical analysis of this technology revealed a detection sensitivity of 1 CTC per litre of blood (~1,000 times better than that of pre-existing assays), with CTCs being detected in 27 (96.4%) of 28 patients with melanoma and 0 of 19 individuals without cancer131. Considering the current stage and speed of technological development and understanding of tumour cell biological and physical features, in vivo detection of CTCs for early cancer detection might soon be feasible. Multidisciplinary collaboration between cancer researchers, physicists, bioengineers and clinicians, both in the academic and industry settings, will be required to achieve this ambitious goal.
Molecular characterization of CTCs
In addition to increasing our ability to isolate and/or detect CTCs, further molecular characterization of the CTCs might enhance the possibility of using these cells to distinguish clinically significant and non-significant, indolent cancers. Identifying different subsets of CTCs, for example, distinguishing between dormant and proliferative CTCs and CTCs associated with different immune cells137, might be clinically useful. In practice, this approach could involve the addition of immune-specific markers to CTC cluster analyses and/or RNA sequencing of CTC clusters versus single CTCs to identify which types of immune cells associate with CTCs82,138 and what effects these interactions have on specific cellular pathways and, thus, on metastatic potential. Standard immunofluorescence analysis is limited by the number of markers that can be measured simultaneously; therefore, novel technologies, such as multiplex immunofluorescence analysis (for example, the MACSima imaging cyclic staining technology)139 or mass cytometry, might be useful. Imaging mass cytometry exploits metal isotopes conjugated to antibodies for the simultaneous analysis of up to 42 markers140. Thus, this platform has the potential to provide additional phenotypic information on individual CTCs, CTC–CTC clusters and CTC–immune cell clusters to further refine their clinical utility. Alternative technologies including imaging cytometry, which combines principles of flow cytometry and fluorescent microscopy, also offer the potential to measure multiple molecular markers on CTCs and might, therefore, enable the identification of novel biomarkers for aggressive cancers in the early detection setting. Single-cell analysis technologies will need to be developed further to reduce their cost and complexity in order to facilitate their clinical application. In addition, the ex vivo culture of CTCs will widen the window of opportunity for molecular and phenotypic analyses and can provide information on the therapeutic vulnerabilities of cancer cells in individual patients141,142. Such molecular investigations might also help to identify markers to be used for in vivo detection of CTCs using a sensor device.
Combining CTCs with other biomarkers for early cancer detection
Blood is a rich source for cancer biomarker discovery. Taking advantage of blood sampling, the utility of CTCs as an early cancer detection tool might be further improved through combinations with other blood-based biomarkers, including circulating proteins, circulating cell-free tumour DNA (ctDNA), microRNAs (miRNAs), extracellular vesicles and immune cell subsets, each of which has advantages and disadvantages relative to CTCs (Table 2).
Plasma and serum proteins have long been explored and used for early cancer detection143. For example, PSA has a crucial role in prostate cancer detection although, owing to a lack of specificity, PSA testing can lead to overdiagnosis, particularly of indolent cancers144. In the aforementioned study exploring the potential for diagnosing aggressive prostate cancers based on CTC detection using the Parsortix system, CTCs and serum PSA had similar diagnostic accuracy102. Moreover, combining both CTC and PSA analyses substantially increased test accuracy102.
High-throughput targeted DNA methylation sequencing of plasma ctDNA has shown promise in cancer diagnosis145,146, with the potential for early multi-cancer detection as well as prediction of the primary tumour site147,148. miRNAs have also shown potential as early cancer detection tools149 based on the fact that their expression profile is often dysregulated in cancer150. A combination of these biomarkers alongside CTC detection might improve test sensitivity and specificity. For example, the combination of CTC detection and ctDNA quantification has shown increased sensitivity (compared with each biomarker alone) for predicting disease-free survival in patients with early stage triple-negative breast cancer151. This combination of CTC and ctDNA measurements might also be useful in the management of CRC152 and in detection of primary lung cancers153.
The antitumour immune response can be initiated early in cancer development154, and changes in the proportions of peripheral blood leukocyte subsets have been reported as potential biomarkers for early cancer detection155,156. Whether cancer cell dissemination (that is, CTCs) affects the immune response and peripheral blood leukocyte composition remains to be determined, although combining CTC detection and phenotyping of circulating immune cells could potentially also improve the accuracy of early cancer detection tests. These examples highlight the promise of alternative circulating biomarkers and their combination with CTCs in early cancer diagnosis.
In addition to these circulating biomarkers, other non-invasive technologies, such as urinary biomarker assays and cancer imaging, are already in clinical use for early cancer detection. Urinary biomarkers are commonly used in the diagnostic work-up for bladder cancer detection157, including nuclear matrix protein 22 (NMP22)158 and bladder tumour antigen protein159. In addition, a PCA3 (encoding urine prostate cancer antigen 3) mRNA test has been approved by the FDA for use in prostate cancer diagnosis144. However, the combined diagnostic value of CTCs and urinary markers is yet to be investigated. Radiological imaging is also commonly used in cancer diagnosis and population-based screening for breast160 and lung161 cancers. Studies have shown that CTCs have a similar diagnostic accuracy, with higher specificity but inferior sensitivity, to that of various imaging modalities (including mammography, ultrasonography and MRI) in patients with breast cancer107 and a better prognostic accuracy in patients with lung cancer162; therefore, combining these two technologies is likely to improve the detection of clinically relevant cancers163.
CTCs in cancer screening
CTCs are a feature of many types of cancers and, therefore, have great potential to be used as a biomarker for the detection of multiple cancer types. The benefit of a multi-cancer detection approach and the current efforts in this direction have been highlighted in a recent review164. On the basis of the evidence discussed herein, the detection of CTCs is likely to indicate the presence of an aggressive cancer somewhere in the body. Hence, the future development of CTC detection platforms with sufficient sensitivity as well as specificity might provide the first pan-cancer screening technology (Fig. 4).
Wearable devices might eventually be developed that enable non-invasive measurement of circulating tumour cells (CTCs) in a larger volume of blood than is currently feasible to sample routinely in a minimally invasive manner. Such devices could potentially directly image CTCs passing through a blood vessel based on labelling with injectable probes. Integration of clinical and family history with targeted imaging and biomarker analyses would inform localization of the tumour site in individuals with detectable CTCs, ultimately leading to a confirmed diagnosis. If the wearable device does not detect any CTCs, the individual might have to repeat the test at a regular interval (for example, annually) or sooner if they have a high risk of cancer or if prompted by any relevant symptoms.
Before being used for cancer screening in the general population, CTCs might first be applied for more targeted, risk-stratified screening of individuals deemed to be at high risk of developing particular malignancies based on genetic risk factors, lifestyle factors, and/or clinical and family history. Individuals with detectable CTCs could then be referred for further investigations, including organ-targeted imaging (such as mammography for those with an increased risk of breast cancer165 or low-dose CT for those at high risk of lung cancer166), cancer-specific blood-based biomarker testing (serum PSA testing for suspected prostate cancer144, CA19-9 testing for pancreatic cancer167 and ctDNA and/or miRNA analyses for various cancer types145,150,168) or further molecular analysis of CTCs to locate the tumour such as tumour-specific antigen testing, DNA methylation analysis or copy-number aberration profiling169. After integration of the results of such tests with information on patient characteristics, risk factors and suspicious symptoms, a diagnosis might be made with or without biopsy sampling of the suspected tumour site, depending on the feasibility of biopsy sampling and the accuracy of non-invasive biomarkers. Individuals with a negative CTC test result could be considered as being cancer free or having a low risk of cancer and subsequently be re-screened for the presence of CTCs at regular intervals unless they experience any change in symptoms (Fig. 4).
Conclusions
In a rapidly evolving field, huge progress has already been made in understanding the processes involved in early cancer dissemination and metastasis. Micrometastases can be formed early in tumorigenesis and accumulating evidence indicates that CTCs can be detected at early stages in the development of aggressive cancers. Therefore, CTCs have great potential to be used for early cancer detection, enabling the identification of clinically relevant tumours while avoiding overdiagnosis of indolent disease. The current challenge lies in developing technologies to reliably harvest and analyse these scarce but implicative cells. With further technological developments, particularly those enabling highly sensitive detection of CTCs through non-invasive or minimally invasive sampling of a large amount of blood at a convenient time, we expect that CTC analysis will be successfully applied to change the paradigm of early cancer detection and thereby substantially improve outcomes for patients with cancer.
References
WHO. Cancer https://www.who.int/news-room/fact-sheets/detail/cancer (2022).
McPhail, S., Johnson, S., Greenberg, D., Peake, M. & Rous, B. Stage at diagnosis and early mortality from cancer in England. Br. J. Cancer 112, S108–S115 (2015).
Dolly, S. O. et al. The effectiveness of the Guy’s rapid diagnostic clinic (RDC) in detecting cancer and serious conditions in vague symptom patients. Br. J. Cancer 124, 1079–1087 (2021).
Sewell, B. et al. Rapid cancer diagnosis for patients with vague symptoms: a cost-effectiveness study. Br. J. Gen. Pract. 70, e186–e192 (2020).
Patt, D. et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin. Cancer Inform. 4, 1059–1071 (2020).
McCormack, V. & Aggarwal, A. Early cancer diagnosis: reaching targets across whole populations amidst setbacks. Br. J. Cancer 124, 1181–1182 (2021).
Frangioni, J. V. New technologies for human cancer imaging. J. Clin. Oncol. 26, 4012–4021 (2008).
Romero, D. Tracking Cancer in Liquid Biopsies https://media.nature.com/original/magazine-assets/d42859-020-00070-z/d42859-020-00070-z.pdf (2020).
Tellez-Gabriel, M., Knutsen, E. & Perander, M. Current status of circulating tumor cells, circulating tumor DNA, and exosomes in breast cancer liquid biopsies. Int. J. Mol. Sci. https://doi.org/10.3390/ijms21249457 (2020).
Eslami, S. Z., Cortes-Hernandez, L. E., Thomas, F., Pantel, K. & Alix-Panabieres, C. Functional analysis of circulating tumour cells: the KEY to understand the biology of the metastatic cascade. Br. J. Cancer https://doi.org/10.1038/s41416-022-01819-1 (2022).
Suhail, Y. et al. Systems biology of cancer metastasis. Cell Syst. 9, 109–127 (2019).
Braun, S. et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N. Engl. J. Med. 353, 793–802 (2005).
Recasens, A. & Munoz, L. Targeting cancer cell dormancy. Trends Pharmacol. Sci. 40, 128–141 (2019).
Werner, S., Heidrich, I. & Pantel, K. Clinical management and biology of tumor dormancy in breast cancer. Semin. Cancer Biol. 78, 49–62 (2022).
Goldkorn, A. et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 32, 1136–1142 (2014).
Riethdorf, S. et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant “Geparquattro” trial. Clin. Cancer Res. 23, 5384–5393 (2017).
Bidard, F. C. et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J. Natl Cancer Inst. 110, 560–567 (2018).
Aggarwal, C. et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann. Oncol. 24, 420–428 (2013).
Abdalla, T. S. A. et al. Prognostic value of preoperative circulating tumor cells counts in patients with UICC stage I-IV colorectal cancer. PLoS ONE 16, e0252897 (2021).
Rink, M. et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur. Urol. 61, 810–817 (2012).
Gazzaniga, P. et al. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int. J. Cancer 135, 1978–1982 (2014).
Grobe, A. et al. Prognostic relevance of circulating tumor cells in blood and disseminated tumor cells in bone marrow of patients with squamous cell carcinoma of the oral cavity. Clin. Cancer Res. 20, 425–433 (2014).
Garrel, R. et al. Circulating tumor cells as a prognostic factor in recurrent or metastatic head and neck squamous cell carcinoma: the CIRCUTEC prospective study. Clin. Chem. 65, 1267–1275 (2019).
Effenberger, K. E. et al. Improved risk stratification by circulating tumor cell counts in pancreatic cancer. Clin. Cancer Res. 24, 2844–2850 (2018).
Srivastava, S. et al. Cancer overdiagnosis: a biological challenge and clinical dilemma. Nat. Rev. Cancer 19, 349–358 (2019).
Heidrich, I., Deitert, B., Werner, S. & Pantel, K. Liquid biopsy for monitoring of tumor dormancy and early detection of disease recurrence in solid tumors. Cancer Metastasis Rev. https://doi.org/10.1007/s10555-022-10075-x (2023).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Fidler, I. J. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J. Natl Cancer Inst. 45, 773–782 (1970).
Montanari, M. et al. Epithelial-mesenchymal transition in prostate cancer: an overview. Oncotarget 8, 35376–35389 (2017).
Yeung, K. T. & Yang, J. Epithelial-mesenchymal transition in tumor metastasis. Mol. Oncol. 11, 28–39 (2017).
Liu, T. et al. Dysregulated expression of Slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast cancer. J. Surg. Oncol. 107, 188–194 (2013).
Tsang, J. Y. et al. P-cadherin and vimentin are useful basal markers in breast cancers. Hum. Pathol. 44, 2782–2791 (2013).
Nitta, T. et al. Prognostic significance of epithelial-mesenchymal transition-related markers in extrahepatic cholangiocarcinoma: comprehensive immunohistochemical study using a tissue microarray. Br. J. Cancer 111, 1363–1372 (2014).
Satelli, A. & Li, S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 68, 3033–3046 (2011).
Yang, J. & Weinberg, R. A. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev. Cell 14, 818–829 (2008).
Zeeshan, R. & Mutahir, Z. Cancer metastasis — tricks of the trade. Bosn. J. Basic Med. Sci. 17, 172–182 (2017).
Donato, C. et al. Hypoxia triggers the intravasation of clustered circulating tumor cells. Cell Rep. 32, 108105 (2020).
Eslami, S. Z., Cortes-Hernandez, L. E. & Alix-Panabieres, C. The metastatic cascade as the basis for liquid biopsy development. Front. Oncol. 10, 1055 (2020).
Follain, G. et al. Hemodynamic forces tune the arrest, adhesion, and extravasation of circulating tumor cells. Dev. Cell 45, 33–52.e12 (2018).
Ward, M. P. et al. Platelets, immune cells and the coagulation cascade; friend or foe of the circulating tumour cell? Mol. Cancer 20, 59 (2021).
Strilic, B. & Offermanns, S. Intravascular survival and extravasation of tumor cells. Cancer Cell 32, 282–293 (2017).
Bartkowiak, K. et al. In vitro modeling of reoxygenation effects on mRNA and protein levels in hypoxic tumor cells upon entry into the bloodstream. Cells https://doi.org/10.3390/cells9051316 (2020).
Osmani, N. et al. Metastatic tumor cells exploit their adhesion repertoire to counteract shear forces during intravascular arrest. Cell Rep. 28, 2491–2500.e5 (2019).
Klein, C. A. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer 9, 302–312 (2009).
Friberg, S. & Mattson, S. On the growth rates of human malignant tumors: implications for medical decision making. J. Surg. Oncol. 65, 284–297 (1997).
Bilous, M. et al. Quantitative mathematical modeling of clinical brain metastasis dynamics in non-small cell lung cancer. Sci. Rep. 9, 13018 (2019).
Heiss, M. M. et al. Individual development and uPA-receptor expression of disseminated tumour cells in bone marrow: a reference to early systemic disease in solid cancer. Nat. Med. 1, 1035–1039 (1995).
Friberg, S. & Nystrom, A. Cancer metastases: early dissemination and late recurrences. Cancer Growth Metastasis 8, 43–49 (2015).
Hüsemann, Y. et al. Systemic spread is an early step in breast cancer. Cancer Cell 13, 58–68 (2008).
Sanger, N. et al. Disseminated tumor cells in the bone marrow of patients with ductal carcinoma in situ. Int. J. Cancer 129, 2522–2526 (2011).
Banys, M. et al. Hematogenous and lymphatic tumor cell dissemination may be detected in patients diagnosed with ductal carcinoma in situ of the breast. Breast Cancer Res. Treat. 131, 801–808 (2012).
Pantel, K. et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J. Natl Cancer Inst. 85, 1419–1424 (1993).
Ilie, M. et al. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PLoS ONE 9, e111597 (2014).
Rhim, A. D. et al. EMT and dissemination precede pancreatic tumor formation. Cell 148, 349–361 (2012).
Hu, Z. & Curtis, C. Looking backward in time to define the chronology of metastasis. Nat. Commun. 11, 3213 (2020).
Hu, Z. et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 51, 1113–1122 (2019).
Hu, Z., Li, Z., Ma, Z. & Curtis, C. Multi-cancer analysis of clonality and the timing of systemic spread in paired primary tumors and metastases. Nat. Genet. 52, 701–708 (2020).
Pavlidis, N. & Pentheroudakis, G. Cancer of unknown primary site. Lancet 379, 1428–1435 (2012).
Phan, T. G. & Croucher, P. I. The dormant cancer cell life cycle. Nat. Rev. Cancer 20, 398–411 (2020).
Agarwal, P. K. et al. Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 112, 307–314 (2008).
Venclovas, Z., Jievaltas, M. & Milonas, D. Significance of time until PSA recurrence after radical prostatectomy without neo- or adjuvant treatment to clinical progression and cancer-related death in high-risk prostate cancer patients. Front. Oncol. 9, 1286 (2019).
Harper, K. L. et al. Mechanism of early dissemination and metastasis in Her2+ mammary cancer. Nature 540, 588–592 (2016).
Hosseini, H. et al. Early dissemination seeds metastasis in breast cancer. Nature 540, 552–558 (2016).
Klein, C. A. Cancer progression and the invisible phase of metastatic colonization. Nat. Rev. Cancer 20, 681–694 (2020).
Sosa, M. S., Bragado, P. & Aguirre-Ghiso, J. A. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat. Rev. Cancer 14, 611–622 (2014).
Klein, C. A. Framework models of tumor dormancy from patient-derived observations. Curr. Opin. Genet. Dev. 21, 42–49 (2011).
Nicolazzo, C. et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 6, 31726 (2016).
Wang, L., Lankhorst, L. & Bernards, R. Exploiting senescence for the treatment of cancer. Nat. Rev. Cancer 22, 340–355 (2022).
Singh, D. K., Patel, V. G., Oh, W. K. & Aguirre-Ghiso, J. A. Prostate cancer dormancy and reactivation in bone marrow. J. Clin. Med. https://doi.org/10.3390/jcm10122648 (2021).
Ashworth, T. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust. Med. J. 14, 146–147 (1869).
Shen, Z., Wu, A. & Chen, X. Current detection technologies for circulating tumor cells. Chem. Soc. Rev. 46, 2038–2056 (2017).
FDA. Approval Notification for Cell Search Technology https://www.accessdata.fda.gov/cdrh_docs/pdf10/k103502.pdf (2010).
Hyun, K. A. et al. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 7, 24677–24687 (2016).
Xu, L. et al. Optimization and evaluation of a novel size based circulating tumor cell isolation system. PLoS ONE 10, e0138032 (2015).
Xu, L. et al. The novel association of circulating tumor cells and circulating megakaryocytes with prostate cancer prognosis. Clin. Cancer Res. 23, 5112–5122 (2017).
Hofman, V. et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 23, 30–38 (2012).
Zhou, M. D. et al. Separable bilayer microfiltration device for viable label-free enrichment of circulating tumour cells. Sci. Rep. 4, 7392 (2014).
Clawson, G. A. et al. Circulating tumor cells in melanoma patients. PLoS ONE 7, e41052 (2012).
Guan, X. et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun. 39, 1 (2019).
Diamantopoulou, Z. et al. The metastatic spread of breast cancer accelerates during sleep. Nature 607, 156–162 (2022).
Szczerba, B. M. et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature 566, 553–557 (2019).
Fabisiewicz, A. & Grzybowska, E. CTC clusters in cancer progression and metastasis. Med. Oncol. 34, 12 (2017).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Cheung, K. J. & Ewald, A. J. A collective route to metastasis: seeding by tumor cell clusters. Science 352, 167–169 (2016).
Cheung, K. J., Gabrielson, E., Werb, Z. & Ewald, A. J. Collective invasion in breast cancer requires a conserved basal epithelial program. Cell 155, 1639–1651 (2013).
Gkountela, S. et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell 176, 98–112.e14 (2019).
Egan, K. et al. Platelet adhesion and degranulation induce pro-survival and pro-angiogenic signalling in ovarian cancer cells. PLoS ONE 6, e26125 (2011).
Hong, Y., Fang, F. & Zhang, Q. Circulating tumor cell clusters: what we know and what we expect (Review). Int. J. Oncol. 49, 2206–2216 (2016).
Aceto, N. et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158, 1110–1122 (2014).
Peeters, D. J. et al. Circulating tumour cells and lung microvascular tumour cell retention in patients with metastatic breast and cervical cancer. Cancer Lett. 356, 872–879 (2015).
McDaniel, A. S. et al. Phenotypic diversity of circulating tumour cells in patients with metastatic castration-resistant prostate cancer. BJU Int. 120, E30–E44 (2017).
Giesing, M., Driesel, G., Molitor, D. & Suchy, B. Molecular phenotyping of circulating tumour cells in patients with prostate cancer: prediction of distant metastases. BJU Int. 110, E1202–E1211 (2012).
Suo, Y. et al. Proportion of circulating tumor cell clusters increases during cancer metastasis. Cytom. A 91, 250–253 (2017).
Kozminsky, M. et al. Detection of CTC clusters and a dedifferentiated RNA-expression survival signature in prostate cancer. Adv. Sci. 6, 1801254 (2019).
Elazezy, M. et al. Emerging insights into keratin 16 expression during metastatic progression of breast cancer. Cancers https://doi.org/10.3390/cancers13153869 (2021).
Rangel-Pozzo, A. et al. Genomic analysis of localized high-risk prostate cancer circulating tumor cells at the single-cell level. Cells https://doi.org/10.3390/cells9081863 (2020).
Keller, L. & Pantel, K. Unravelling tumour heterogeneity by single-cell profiling of circulating tumour cells. Nat. Rev. Cancer 19, 553–567 (2019).
Chimonidou, M., Strati, A., Malamos, N., Georgoulias, V. & Lianidou, E. S. SOX17 promoter methylation in circulating tumor cells and matched cell-free DNA isolated from plasma of patients with breast cancer. Clin. Chem. 59, 270–279 (2013).
Chimonidou, M. et al. DNA methylation of tumor suppressor and metastasis suppressor genes in circulating tumor cells. Clin. Chem. 57, 1169–1177 (2011).
Kang, B. J. et al. Circulating tumor cell number is associated with primary tumor volume in patients with lung adenocarcinoma. Tuberc. Respir. Dis. 83, 61–70 (2020).
Xu, L. et al. Noninvasive detection of clinically significant prostate cancer using circulating tumor cells. J. Urol. 203, 73–82 (2020).
Muller, C. et al. Hematogenous dissemination of glioblastoma multiforme. Sci. Transl. Med. 6, 247ra101 (2014).
Alix-Panabieres, C. & Pantel, K. Challenges in circulating tumour cell research. Nat. Rev. Cancer 14, 623–631 (2014).
Massague, J. & Obenauf, A. C. Metastatic colonization by circulating tumour cells. Nature 529, 298–306 (2016).
Trapp, E. et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J. Natl Cancer Inst. 111, 380–387 (2019).
Shao, X. et al. A comprehensive comparison of circulating tumor cells and breast imaging modalities as screening tools for breast cancer in Chinese women. Front. Oncol. 12, 890248 (2022).
Krol, I. et al. Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 125, 23–27 (2021).
Tsai, W. S. et al. Novel circulating tumor cell assay for detection of colorectal adenomas and cancer. Clin. Transl. Gastroenterol. 10, e00088 (2019).
Bork, U. et al. Circulating tumour cells and outcome in non-metastatic colorectal cancer: a prospective study. Br. J. Cancer 112, 1306–1313 (2015).
Loh, J. et al. Circulating tumor cell detection in high-risk non-metastatic prostate cancer. J. Cancer Res. Clin. Oncol. 140, 2157–2162 (2014).
Wankhede, D., Grover, S. & Hofman, P. Circulating tumor cells as a predictive biomarker in resectable lung cancer: a systematic review and meta-analysis. Cancers https://doi.org/10.3390/cancers14246112 (2022).
Ankeny, J. S. et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br. J. Cancer 114, 1367–1375 (2016).
Zumsteg, Z. S. & Zelefsky, M. J. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: the standard of care? Lancet Oncol. 13, e259–e269 (2012).
Zumsteg, Z. S. et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur. Urol. 64, 895–902 (2013).
Ried, K., Eng, P. & Sali, A. Screening for circulating tumour cells allows early detection of cancer and monitoring of treatment effectiveness: an observational study. Asian Pac. J. Cancer Prev. 18, 2275–2285 (2017).
Marquette, C. H. et al. Circulating tumour cells as a potential biomarker for lung cancer screening: a prospective cohort study. Lancet Respir. Med. 8, 709–716 (2020).
Welch, H. G. & Black, W. C. Overdiagnosis in cancer. J. Natl Cancer Inst. 102, 605–613 (2010).
Ma, J. et al. Artificial intelligence based on blood biomarkers including CTCs predicts outcomes in epithelial ovarian cancer: a prospective study. Onco Targets Ther. 14, 3267–3280 (2021).
Chemi, F. et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat. Med. 25, 1534–1539 (2019).
Liu, X. et al. Detection of CTCs in portal vein was associated with intrahepatic metastases and prognosis in patients with advanced pancreatic cancer. J. Cancer 9, 2038–2045 (2018).
Deneve, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. Clin. Chem. 59, 1384–1392 (2013).
Cortes-Hernandez, L. E. et al. Do malignant cells sleep at night? Genome Biol. 21, 276 (2020).
Dauvilliers, Y., Thomas, F. & Alix-Panabieres, C. Dissemination of circulating tumor cells at night: role of sleep or circadian rhythm? Genome Biol. 23, 214 (2022).
Scher, H. I. et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol. 10, 233–239 (2009).
Kim, T. H. et al. A temporary indwelling intravascular aphaeretic system for in vivo enrichment of circulating tumor cells. Nat. Commun. 10, 1478 (2019).
Andree, K. C. et al. Toward a real liquid biopsy in metastatic breast and prostate cancer: diagnostic LeukApheresis increases CTC yields in a European prospective multicenter study (CTCTrap). Int. J. Cancer 143, 2584–2591 (2018).
Lambros, M. B. et al. Single-cell analyses of prostate cancer liquid biopsies acquired by apheresis. Clin. Cancer Res. 24, 5635–5644 (2018).
Saucedo-Zeni, N. et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 41, 1241–1250 (2012).
Gorges, T. M. et al. Enumeration and molecular characterization of tumor cells in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. Clin. Cancer Res. 22, 2197–2206 (2016).
Galanzha, E. I. et al. In vivo liquid biopsy using Cytophone platform for photoacoustic detection of circulating tumor cells in patients with melanoma. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aat5857 (2019).
Nicolson, F. et al. Non-invasive in vivo imaging of cancer using surface-enhanced spatially offset Raman Spectroscopy (SESORS). Theranostics 9, 5899–5913 (2019).
Miwa, S. et al. Real-time in vivo confocal fluorescence imaging of prostate cancer bone-marrow micrometastasis development at the cellular level in nude mice. J. Cell Biochem. 117, 2533–2537 (2016).
White, M. D., Zhao, Z. W. & Plachta, N. In vivo imaging of single mammalian cells in development and disease. Trends Mol. Med. 24, 278–293 (2018).
Galanzha, E. I., Shashkov, E. V., Spring, P. M., Suen, J. Y. & Zharov, V. P. In vivo, noninvasive, label-free detection and eradication of circulating metastatic melanoma cells using two-color photoacoustic flow cytometry with a diode laser. Cancer Res. 69, 7926–7934 (2009).
Nedosekin, D. A., Sarimollaoglu, M., Ye, J. H., Galanzha, E. I. & Zharov, V. P. In vivo ultra-fast photoacoustic flow cytometry of circulating human melanoma cells using near-infrared high-pulse rate lasers. Cytom. A 79, 825–833 (2011).
Pereira-Veiga, T., Schneegans, S., Pantel, K. & Wikman, H. Circulating tumor cell-blood cell crosstalk: biology and clinical relevance. Cell Rep. 40, 111298 (2022).
Brechbuhl, H. M. et al. Analysis of circulating breast cancer cell heterogeneity and interactions with peripheral blood mononuclear cells. Mol. Carcinog. 59, 1129–1139 (2020).
Kinkhabwala, A. et al. MACSima imaging cyclic staining (MICS) technology reveals combinatorial target pairs for CAR T cell treatment of solid tumors. Sci. Rep. 12, 1911 (2022).
Kay, A. W., Strauss-Albee, D. M. & Blish, C. A. Application of mass cytometry (CyTOF) for functional and phenotypic analysis of natural killer cells. Methods Mol. Biol. 1441, 13–26 (2016).
Donato, C., Szczerba, B. M., Scheidmann, M. C., Castro-Giner, F. & Aceto, N. Micromanipulation of circulating tumor cells for downstream molecular analysis and metastatic potential assessment. J. Vis. Exp. https://doi.org/10.3791/59677 (2019).
Koch, C. et al. Characterization of circulating breast cancer cells with tumorigenic and metastatic capacity. EMBO Mol. Med. 12, e11908 (2020).
Landegren, U. & Hammond, M. Cancer diagnostics based on plasma protein biomarkers: hard times but great expectations. Mol. Oncol. 15, 1715–1726 (2021).
Boerrigter, E., Groen, L. N., Van Erp, N. P., Verhaegh, G. W. & Schalken, J. A. Clinical utility of emerging biomarkers in prostate cancer liquid biopsies. Expert Rev. Mol. Diagn. 20, 219–230 (2020).
Liang, W. et al. Non-invasive diagnosis of early-stage lung cancer using high-throughput targeted DNA methylation sequencing of circulating tumor DNA (ctDNA). Theranostics 9, 2056–2070 (2019).
Luo, H. et al. Circulating tumor DNA methylation profiles enable early diagnosis, prognosis prediction, and screening for colorectal cancer. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aax7533 (2020).
Neal, R. D. et al. Cell-free DNA-based multi-cancer early detection test in an asymptomatic screening population (NHS-Galleri): design of a pragmatic, prospective randomised controlled trial. Cancers https://doi.org/10.3390/cancers14194818 (2022).
Tang, W. H. W. et al. Performance of a targeted methylation-based multi-cancer early detection test by race and ethnicity. Prev. Med. 167, 107384 (2023).
Zhang, H. et al. Plasma miR-145, miR-20a, miR-21 and miR-223 as novel biomarkers for screening early-stage non-small cell lung cancer. Oncol. Lett. 13, 669–676 (2017).
Hamam, R. et al. Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis. 8, e3045 (2017).
Radovich, M. et al. Association of circulating tumor DNA and circulating tumor cells after neoadjuvant chemotherapy with disease recurrence in patients with triple-negative breast cancer: preplanned secondary analysis of the BRE12-158 randomized clinical trial. JAMA Oncol. 6, 1410–1415 (2020).
Kidess-Sigal, E. et al. Enumeration and targeted analysis of KRAS, BRAF and PIK3CA mutations in CTCs captured by a label-free platform: comparison to ctDNA and tissue in metastatic colorectal cancer. Oncotarget 7, 85349–85364 (2016).
Moon, S. M. et al. Clinical utility of combined circulating tumor cell and circulating tumor DNA assays for diagnosis of primary lung cancer. Anticancer Res. 40, 3435–3444 (2020).
Pylaeva, E. et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 40, 111171 (2022).
Prodromidou, A. et al. The diagnostic efficacy of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in ovarian cancer. Inflamm. Res. 66, 467–475 (2017).
Ozyalvacli, G. et al. Diagnostic and prognostic importance of the neutrophil lymphocyte ratio in breast cancer. Asian Pac. J. Cancer Prev. 15, 10363–10366 (2014).
Chakraborty, A., Dasari, S., Long, W. & Mohan, C. Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am. J. Cancer Res. 9, 1104–1117 (2019).
Soloway, M. S. et al. Use of a new tumor marker, urinary NMP22, in the detection of occult or rapidly recurring transitional cell carcinoma of the urinary tract following surgical treatment. J. Urol. 156, 363–367 (1996).
Guo, A. et al. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: a meta-analysis. Can. Urol. Assoc. J. 8, E347–E352 (2014).
Michaels, E., Worthington, R. O. & Rusiecki, J. Breast cancer: risk assessment, screening, and primary prevention. Med. Clin. North Am. 107, 271–284 (2023).
Adams, S. J. et al. Lung cancer screening. Lancet 401, 390–408 (2023).
Zhang, F. et al. 18F-FDG PET/CT and circulating tumor cells in treatment-naive patients with non-small-cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 48, 3250–3259 (2021).
Gao, Y. et al. Enhancing the screening efficiency of breast cancer by combining conventional medical imaging examinations with circulating tumor cells. Front. Oncol. 11, 643003 (2021).
Kisiel, J. B. et al. Multicancer early detection test: preclinical, translational, and clinical evidence-generation plan and provocative questions. Cancer 128, 861–874 (2022).
Fiorica, J. V. Breast cancer screening, mammography, and other modalities. Clin. Obstet. Gynecol. 59, 688–709 (2016).
Heuvelmans, M. A., Groen, H. J. & Oudkerk, M. Early lung cancer detection by low-dose CT screening: therapeutic implications. Expert Rev. Respir. Med. 11, 89–100 (2017).
Ge, L. et al. Comparing the diagnostic accuracy of five common tumour biomarkers and CA19-9 for pancreatic cancer: a protocol for a network meta-analysis of diagnostic test accuracy. BMJ Open 7, e018175 (2017).
Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359, 926–930 (2018).
Riebensahm, C. et al. Clonality of circulating tumor cells in breast cancer brain metastasis patients. Breast Cancer Res. 21, 101 (2019).
Ntouroupi, T. G. et al. Detection of circulating tumour cells in peripheral blood with an automated scanning fluorescence microscope. Br. J. Cancer 99, 789–795 (2008).
Wendel, M. et al. Fluid biopsy for circulating tumor cell identification in patients with early-and late-stage non-small cell lung cancer: a glimpse into lung cancer biology. Phys. Biol. 9, 016005 (2012).
Kulemann, B. et al. Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas 44, 547–550 (2015).
Kang, H. M. et al. Circulating tumor cells detected by lab-on-a-disc: role in early diagnosis of gastric cancer. PLoS ONE 12, e0180251 (2017).
Gupta, P. et al. Analytical validation of the CellMax platform for early detection of cancer by enumeration of rare circulating tumor cells. J. Circ. Biomark. 8, 1849454419899214 (2019).
Karimi, N., Oloomi, M. & Orafa, Z. Circulating tumor cells detection in patients with early breast cancer using MACS immunomagnetic flow cytometry. Avicenna J. Med. Biotechnol. 12, 148–156 (2020).
Sharma, S. et al. Circulating tumor cell isolation, culture, and downstream molecular analysis. Biotechnol. Adv. 36, 1063–1078 (2018).
Chemi, F., Mohan, S. & Brady, G. Circulating tumour cells in lung cancer. Recent Results Cancer Res. 215, 105–125 (2020).
Huang, Z., Ma, L., Huang, C., Li, Q. & Nice, E. C. Proteomic profiling of human plasma for cancer biomarker discovery. Proteomics https://doi.org/10.1002/pmic.201600240 (2017).
Fuzery, A. K., Levin, J., Chan, M. M. & Chan, D. W. Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin. Proteom. 10, 13 (2013).
Bjorkesten, J. et al. Stability of proteins in dried blood spot biobanks. Mol. Cell Proteom. 16, 1286–1296 (2017).
Devine, P. L. High dose hook effect and sample carryover in carcinoembryonic antigen assay performed on the Boehringer-Mannheim ES-300 automated immunoassay system. Eur. J. Clin. Chem. Clin. Biochem. 34, 573–574 (1996).
Chen, M. & Zhao, H. Next-generation sequencing in liquid biopsy: cancer screening and early detection. Hum. Genomics 13, 34 (2019).
Tivey, A., Church, M., Rothwell, D., Dive, C. & Cook, N. Circulating tumour DNA — looking beyond the blood. Nat. Rev. Clin. Oncol. 19, 600–612 (2022).
Merker, J. D. et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. J. Clin. Oncol. 36, 1631–1641 (2018).
Katsman, E. et al. Detecting cell-of-origin and cancer-specific methylation features of cell-free DNA from nanopore sequencing. Genome Biol. 23, 158 (2022).
Liu, W. et al. Response prediction and risk stratification of patients with rectal cancer after neoadjuvant therapy through an analysis of circulating tumour DNA. EBioMedicine 78, 103945 (2022).
Mack, P. C. et al. Circulating tumor DNA kinetics predict progression-free and overall survival in EGFR TKI-treated patients with EGFR-mutant NSCLC (SWOG S1403). Clin. Cancer Res. 28, 3752–3760 (2022).
Fiala, C. & Diamandis, E. P. Utility of circulating tumor DNA in cancer diagnostics with emphasis on early detection. BMC Med. 16, 166 (2018).
Phallen, J. et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. https://doi.org/10.1126/scitranslmed.aan2415 (2017).
Heidrich, I. & Pantel, K. Liquid biopsy: blood-based analyses of circulating cell-free DNA in xenografts. EMBO Mol. Med. 14, e16326 (2022).
Danesi, R. et al. What do we need to obtain high quality circulating tumor DNA (ctDNA) for routine diagnostic test in oncology? — Considerations on pre-analytical aspects by the IFCC workgroup cfDNA. Clin. Chim. Acta 520, 168–171 (2021).
Jaiswal, S. & Ebert, B. L. Clonal hematopoiesis in human aging and disease. Science https://doi.org/10.1126/science.aan4673 (2019).
Lu, J. et al. MicroRNA expression profiles classify human cancers. Nature 435, 834–838 (2005).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl Acad. Sci. USA 105, 10513–10518 (2008).
Schwarzenbach, H., Nishida, N., Calin, G. A. & Pantel, K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat. Rev. Clin. Oncol. 11, 145–156 (2014).
Ho, P. T. B., Clark, I. M. & Le, L. T. T. MicroRNA-based diagnosis and therapy. Int. J. Mol. Sci. https://doi.org/10.3390/ijms23137167 (2022).
Becker, N. & Lockwood, C. M. Pre-analytical variables in miRNA analysis. Clin. Biochem. 46, 861–868 (2013).
Yu, W. et al. Exosome-based liquid biopsies in cancer: opportunities and challenges. Ann. Oncol. 32, 466–477 (2021).
Zhu, L. et al. Isolation and characterization of exosomes for cancer research. J. Hematol. Oncol. 13, 152 (2020).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Yu, D. et al. Exosomes as a new frontier of cancer liquid biopsy. Mol. Cancer 21, 56 (2022).
Acknowledgements
K.P. has received funding from the European Innovative Medicines Initiative (IMI) research project CANCER-ID (115749-CANCER-ID); the European Union Horizon 2020 research and innovation programme, under the Marie Skłodowska-Curie grant agreement No. 765492 and the ERA-NET EU/TRANSCAN-2 Third Joint Transnational Call (JTC 2016) project PROLIPSY; the Deutsche Forschungsgemeinschaft (DFG; Priority Program SPP2084: µBone); and the ERC Advanced Investigator Grant INJURMET (agreement No. 834974). Y.-J.L. has received funding from Orchid, ANGLE and Prostate Cancer UK (MA-CT20–011).
Author information
Authors and Affiliations
Contributions
R.L. and Y.-J.L. researched data for the article and wrote the manuscript. R.L., K.P. and Y.-J.L. made substantial contributions to the discussion of content. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
Y.-J.L. has received commercial research grants from ANGLE. K.P. has received personal fees from Agena, Illumina, and Menarini outside the submitted work and has a patent pending with the European Patent Office (application No. 18705153.7; PCT/EP2018/054052; Method of Detecting Cancer or Cancer Cells). The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Clinical Oncology thanks R. Henrique, who co-reviewed with J. Lobo; J.-Y. Pierga; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Accelerating detection of disease challenge: https://www.ukri.org/what-we-offer/our-main-funds/ukri-challenge-fund/ageing-society/accelerating-detection-of-disease-challenge/
Cancer Grand Challenge investigating Dormancy: https://cancergrandchallenges.org/challenges/dormancy-2017
Cancer Moonshot: https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative
ClinicalTrials.gov: https://clinicaltrials.gov/ct2/home
European Liquid Biopsy Society: www.elbs.eu
Europe’s Beating Cancer Plan: https://ec.europa.eu/health/sites/health/files/non_communicable_diseases/docs/eu_cancer-plan_en.pdf
PANCAID: https://www.pancaid-project.eu/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lawrence, R., Watters, M., Davies, C.R. et al. Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol 20, 487–500 (2023). https://doi.org/10.1038/s41571-023-00781-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-023-00781-y
This article is cited by
-
Liquid biopsy techniques and lung cancer: diagnosis, monitoring and evaluation
Journal of Experimental & Clinical Cancer Research (2024)
-
Orthotopic model for the analysis of melanoma circulating tumor cells
Scientific Reports (2024)
-
Poor patient outcome correlates with active engulfment of cytokeratin positive CTCs within cancer-associated monocyte population in lung cancer
Clinical & Experimental Metastasis (2024)
-
miRNAs in pancreatic cancer progression and metastasis
Clinical & Experimental Metastasis (2024)
-
Can our experience with surveillance for inherited pancreatic cancer help to identify early pancreatic cancer in the general population?
Familial Cancer (2024)