Featured
-
-
News & Views |
 Discovering cryptic natural products by substrate manipulation
Discovering cryptic natural products by substrate manipulation
Cryptic halogenation reactions result in natural products with diverse structural motifs and bioactivities. However, these halogenated species are difficult to detect with current analytical methods because the final products are often not halogenated. An approach to identify products of cryptic halogenation using halide depletion has now been discovered, opening up space for more effective natural product discovery.
- Ludek Sehnal
- , Libera Lo Presti
- & Nadine Ziemert
-
News & Views |
 Ribozyme for stabilized SAM analogue modifies RNA in cells
Ribozyme for stabilized SAM analogue modifies RNA in cells
Site-specific modification of RNA in cells is crucial for analysis and functional investigations. Natural enzymes that promote RNA methylation using S-adenosyl-l-methionine (SAM) exist, but leveraging these proteins for RNA modification is limited by cell permeability, stability and specificity of their substrates. Now, a de novo ribozyme that acts on a stabilized and cell-permeable SAM analogue enables site-specific RNA modification with a click handle in living cells.
- Nicolas V. Cornelissen
- & Andrea Rentmeister
-
News & Views |
 Engineering yeast to produce plant-derived anti-obesity agent
Engineering yeast to produce plant-derived anti-obesity agent
Plants produce a wide range of compounds with important bioactivities. Celastrol, an anti-obesity agent found in the root of certain plants, can now be produced de novo in yeast.
- Jens Nielsen
-
Article |
 Enhanced active-site electric field accelerates enzyme catalysis
Enhanced active-site electric field accelerates enzyme catalysis
The design and improvement of enzymes based on physical principles remain challenging. Now, the vibrational Stark effect has been used to demonstrate how an electrostatic model can unify the catalytic effects of distinct chemical forces in a quantitative manner and guide the design of enzyme variants that outperform their natural counterpart.
- Chu Zheng
- , Zhe Ji
- & Steven G. Boxer
-
Article
| Open Access Expanding the substrate scope of pyrrolysyl-transfer RNA synthetase enzymes to include non-α-amino acids in vitro and in vivo
Expanding the substrate scope of pyrrolysyl-transfer RNA synthetase enzymes to include non-α-amino acids in vitro and in vivo
Ribosomal incorporation of non-α-amino acid monomers into proteins is largely restricted to in vitro translation. Now, pyrrolysyl-transfer RNA synthetase variants have been shown to acylate tRNAs with α-thio acids, malonic acids, and N-formyl amino acids. This work represents a key step towards the programmed ribosomal synthesis of sequence-defined non-protein polymers in cellulo.
- Riley Fricke
- , Cameron V. Swenson
- & Alanna Schepartz
-
Article |
 Activity-based directed evolution of a membrane editor in mammalian cells
Activity-based directed evolution of a membrane editor in mammalian cells
Cellular membranes contain numerous lipids, and efforts to understand the biological functions of individual lipids demand approaches for controlled modulation of membrane composition in situ. Now, click chemistry-based directed evolution of a microbial phospholipase within mammalian cells affords an editor for optogenetic, targeted modification of phospholipids in cell membranes.
- Reika Tei
- , Saket R. Bagde
- & Jeremy M. Baskin
-
Article |
 Biosynthesis-guided discovery reveals enteropeptins as alternative sactipeptides containing N-methylornithine
Biosynthesis-guided discovery reveals enteropeptins as alternative sactipeptides containing N-methylornithine
Enteropeptins are peptide natural products produced by the gut microbe Enterococcus cecorum. Now, the structure, biosynthesis and function of enteropeptins have been determined. After ribosomal biosynthesis, enteropeptins are post-translationally modified in three reactions carried out by a radical S-adenosylmethionine enzyme, an Mn2+-dependent arginase, and an Fe–S-containing methyltransferase, respectively, to form the N-methylornithine-containing peptide natural products.
- Kenzie A. Clark
- , Brett C. Covington
- & Mohammad R. Seyedsayamdost
-
Article |
 A modular XNAzyme cleaves long, structured RNAs under physiological conditions and enables allele-specific gene silencing
A modular XNAzyme cleaves long, structured RNAs under physiological conditions and enables allele-specific gene silencing
Oligonucleotide catalysts such as ribozymes and DNAzymes can cleave RNA efficiently and specifically but are typically dependent on high concentrations of divalent cations, limiting their biological applications. A modular XNAzyme catalyst composed of 2′-deoxy-2′-fluoro-β-d-arabino nucleic acid (FANA) has now been developed that can cleave long (>5 kb), highly structured mRNAs under physiological conditions and enables allele-specific catalytic RNA knockdown inside cells.
- Alexander I. Taylor
- , Christopher J. K. Wan
- & Philipp Holliger
-
Article |
 Biosynthesis and characterization of fuscimiditide, an aspartimidylated graspetide
Biosynthesis and characterization of fuscimiditide, an aspartimidylated graspetide
The biosynthesis of fuscimiditide, a ribosomally synthesized post-translationally modified peptide, has now been reported. Heterologous expression and analysis of fuscimiditide showed it contained two side-chain–side-chain ester linkages and an aspartimide in its backbone. The aspartimide moiety is unexpectedly stable, suggesting this structure is the intended natural product.
- Hader E. Elashal
- , Joseph D. Koos
- & A. James Link
-
News & Views |
 Warhead assembly in a lethal pathogen
Warhead assembly in a lethal pathogen
Malleicyprols are highly reactive polyketides responsible for virulence in some pathogenic bacteria. Now, the enzyme that constructs the cyclopropanol warhead of malleicyprols has been identified. This enzyme could represent a useful target for developing new antivirulence therapeutics.
- Elijah Abraham
- & Rebecca A. Butcher
-
Article
| Open Access Pathogenic bacteria remodel central metabolic enzyme to build a cyclopropanol warhead
Pathogenic bacteria remodel central metabolic enzyme to build a cyclopropanol warhead
Burkholderia pseudomallei is a species of bacteria that poses a global health threat and, more generally, bacteria of the Burkholderia pseudomallei group cause severe diseases that are recalcitrant to treatment with antibiotics. Now, it has been shown how these infamous pathogens repurpose the widespread enzyme BurG to produce a reactive cyclopropanol head group found in the virulence-promoting malleicyprol toxins. Interrupting the synthesis of the cyclopropanol warhead is a potential route for developing antivirulence treatments.
- Felix Trottmann
- , Keishi Ishida
- & Christian Hertweck
-
-
Article |
 Serial crystallography captures dynamic control of sequential electron and proton transfer events in a flavoenzyme
Serial crystallography captures dynamic control of sequential electron and proton transfer events in a flavoenzyme
A reduction reaction is usually equated with an electron transfer reaction. Now, ultrafast time-resolved serial femtosecond X-ray crystallography has enabled the visualization of the stepwise structural changes that occur after electron transfers have been observed in the light-triggered reduction of flavin adenine dinucleotide catalysed by DNA photolyase.
- Manuel Maestre-Reyna
- , Cheng-Han Yang
- & Ming-Daw Tsai
-
Article |
 Fast CO2 hydration kinetics impair heterogeneous but improve enzymatic CO2 reduction catalysis
Fast CO2 hydration kinetics impair heterogeneous but improve enzymatic CO2 reduction catalysis
Carbonic anhydrase enzymatically catalyses CO2 hydration, and its effect on enzymatic and heterogeneous CO2 reduction has now been studied. Through the co-immobilization of carbonic anhydrase, it has been shown that faster CO2 hydration kinetics are beneficial for enzymatic catalysis (using formate dehydrogenase) but detrimental for heterogeneous catalysts, such as gold.
- Samuel J. Cobb
- , Vivek M. Badiani
- & Erwin Reisner
-
Article |
 A dual cellular–heterogeneous catalyst strategy for the production of olefins from glucose
A dual cellular–heterogeneous catalyst strategy for the production of olefins from glucose
A dual cellular-then-heterogeneous catalysis strategy has been used to produce olefins from glucose. 3-Hydroxy acids are made using an engineered microbial host. A hydrolytic step then provides the driving force for fatty acid deoxygenation by simple heterogeneous Lewis acid catalysis. This decarboxylation–dehydration route to olefinic products avoids the need for an additional redox input typically required for deoxygenation of unmodified fatty acids.
- Zhen Q. Wang
- , Heng Song
- & Michelle C. Y. Chang
-
Article |
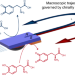 Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers
Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers
Self-propelled artificial chemical swimmers have previously been developed for chemical sensing. Now, hybrid bioelectrochemical swimmers, capable of translating chiral molecular information into macroscopic motion, have been developed. Diastereomeric interactions between enantiopure oligomers immobilized on the swimmer and a chiral molecule present in solution control the trajectory of the device.
- Serena Arnaboldi
- , Gerardo Salinas
- & Alexander Kuhn
-
Article |
 Evolution of dynamical networks enhances catalysis in a designer enzyme
Evolution of dynamical networks enhances catalysis in a designer enzyme
Computationally designed enzymes can be substantially improved by directed evolution. Now, it has been shown that evolution can introduce a dynamic network that selectively tightens the transition-state ensemble, giving rise to a negative activation heat capacity. Targeting such transition state conformational dynamics may expedite de novo enzyme creation.
- H. Adrian Bunzel
- , J. L. Ross Anderson
- & Adrian J. Mulholland
-
Article |
 Efficient Lewis acid catalysis of an abiological reaction in a de novo protein scaffold
Efficient Lewis acid catalysis of an abiological reaction in a de novo protein scaffold
A de novo designed zinc-binding protein has been converted into a highly active, stereoselective catalyst for a hetero-Diels–Alder reaction. Design and directed evolution were used to effectively harness Lewis acid catalysis and create an enzyme more proficient than other reported Diels–Alderases.
- Sophie Basler
- , Sabine Studer
- & Donald Hilvert
-
News & Views |
 Challenging nature’s preference for methylation
Challenging nature’s preference for methylation
Enzymes that methylate using S-adenosyl-l-methionine — nature’s methyliodide — are abundant and often promiscuous; however, a preference for alkylation over methylation has been neither observed in nature nor engineered. Now, carboxymethylation has been demonstrated using engineered methyltransferases.
- Jennifer N. Andexer
- & Andrea Rentmeister
-
News & Views |
 Expanding the reverse transcription toolbox
Expanding the reverse transcription toolbox
Tailor-made reverse transcriptases are used in molecular biology and synthetic genetics. However, re-engineering these enzymes to work with non-natural nucleic acids is difficult and requires powerful directed evolution strategies. Now, an adaptable selection approach has been demonstrated for the evolution of new reverse transcriptases.
- Melanie Henkel
- & Andreas Marx
-
Article |
 Directional conformer exchange in dihydrofolate reductase revealed by single-molecule nanopore recordings
Directional conformer exchange in dihydrofolate reductase revealed by single-molecule nanopore recordings
Single-molecule nanopore measurements have revealed ligand-induced conformational changes in the catalytic cycle of dihydrofolate reductase, and showed that the enzyme adopts distinctive conformers, which have different affinities for substrates and products. Crossing the transition state facilitates conformer exchange, suggesting that the chemical step catalyses the switch between conformers to obtain a more efficient product release.
- Nicole Stéphanie Galenkamp
- , Annemie Biesemans
- & Giovanni Maglia
-
Article |
 A dual transacylation mechanism for polyketide synthase chain release in enacyloxin antibiotic biosynthesis
A dual transacylation mechanism for polyketide synthase chain release in enacyloxin antibiotic biosynthesis
Enacyloxin IIa is an antibiotic, assembled by a modular polyketide synthase, with promising activity against the Gram-negative bacterium Acinetobacter baumannii. Now, it has been shown that the enacyloxin IIa polyketide chain is released via transfer to a separately encoded carrier protein by a non-elongating ketosynthase domain, followed by condensation with 3,4-dihydroxycyclohexane carboxylic acid by a non-ribosomal peptide synthetase condensation domain.
- Joleen Masschelein
- , Paulina K. Sydor
- & Gregory L. Challis
-
Article |
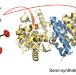 A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor
A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor
Natural hydrogenases exclusively utilize Ni and/or Fe to activate or produce hydrogen. Now, a catalytically active [Mn]-hydrogenase has been prepared by incorporating a synthetic Mn complex into the apoenzyme of [Fe]-Hydrogenase. The semi-synthetic [Mn]-hydrogenase shows higher activity than the corresponding Fe analogue.
- Hui-Jie Pan
- , Gangfeng Huang
- & Xile Hu
-
Article |
 An automated platform for the enzyme-mediated assembly of complex oligosaccharides
An automated platform for the enzyme-mediated assembly of complex oligosaccharides
An automated platform that can synthesize a wide range of complex glycans could greatly facilitate progress in glycoscience. Now, a fully automated process for enzyme-mediated oligosaccharide synthesis has been developed. This process uses glycosyltransferase-catalysed reactions performed in solution, with product purification being accomplished by solid phase extraction using a sulfonate tag.
- Tiehai Li
- , Lin Liu
- & Geert-Jan Boons
-
Article |
 Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy
Streamlining the chemoenzymatic synthesis of complex N-glycans by a stop and go strategy
Preparation of well-defined N-glycans is very demanding, which hampers progress in glycoscience. Now, a biomimetic synthetic approach has been developed in which a readily available bi-antennary glycan can be converted in ten or fewer steps into multi-antennary N-glycans. This approach enables each arm to be uniquely extended by glycosyltransferases to give complex branched N-glycans.
- Lin Liu
- , Anthony R. Prudden
- & Geert-Jan Boons
-
Article |
 Mining the cellular inventory of pyridoxal phosphate-dependent enzymes with functionalized cofactor mimics
Mining the cellular inventory of pyridoxal phosphate-dependent enzymes with functionalized cofactor mimics
A chemical proteomic strategy has now been developed for profiling pyridoxal-phosphate dependent enzymes (PLP-DEs) in cells. Pyridoxal-based probes are phosphorylated in situ and bind to cellular PLP-DEs as cofactor mimics. The method accessed 73% of the Staphylococcus aureus PLP-dependent proteome and annotated uncharacterized proteins as novel PLP-DEs.
- Annabelle Hoegl
- , Matthew B. Nodwell
- & Stephan A. Sieber
-
Article |
 A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue
A designer enzyme for hydrazone and oxime formation featuring an unnatural catalytic aniline residue
An enzyme in which a reactive unnatural amino acid functions as a catalytic residue has now been designed. Embedding an aniline side chain into the promiscuous binding pocket of a multidrug resistance regulator endowed the protein scaffold with new-to-nature activities for hydrazone and oxime formation.
- Ivana Drienovská
- , Clemens Mayer
- & Gerard Roelfes
-
Article |
 Dynamic protein assembly by programmable DNA strand displacement
Dynamic protein assembly by programmable DNA strand displacement
The construction of dynamic protein–DNA nano-assemblies suitable for modulating protein proximities and activities has now been demonstrated. This approach uses DNA strand displacement and can enable control of enzyme activity to be programmed using logic gates. As a demonstration, a split enzyme capable of activating a prodrug is triggered is by cancer-specific miRNAs.
- Rebecca P. Chen
- , Daniel Blackstock
- & Wilfred Chen
-
Article |
 Nonribosomal biosynthesis of backbone-modified peptides
Nonribosomal biosynthesis of backbone-modified peptides
Nonribosomal peptide synthetases (NRPSs) produce vital natural products but have proven recalcitrant to biosynthetic engineering. Now, a combination of yeast surface display and fluorescence-activated cell sorting (FACS) has been used to reprogram an L-Phe-incorporating module for β-Phe. The resulting module is highly selective and functions efficiently in NRPS pathways.
- David L. Niquille
- , Douglas A. Hansen
- & Donald Hilvert
-
Article |
 Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme
Enantioselective, intermolecular benzylic C–H amination catalysed by an engineered iron-haem enzyme
The intermolecular amination of C–H bonds is an enabling transformation for the synthesis of nitrogen-containing molecules; however, developing catalysts for this class of reactions is very challenging. Now, an iron-based enzyme for this reaction has been engineered, demonstrating that a protein can confer a difficult new function upon an otherwise unreactive base metal.
- Christopher K. Prier
- , Ruijie K. Zhang
- & Frances H. Arnold
-
Article |
 Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases
Why copper is preferred over iron for oxygen activation and reduction in haem-copper oxidases
Despite decades of research into heme-copper oxidases, the advantages provided by copper over iron as the non-heme metal has remained unclear. Now, the preference of copper over iron has finally been explained. Copper favours faster electron transfer and higher O–O bond activation, which results in much higher oxidase activity than would be achieved by an iron equivalent.
- Ambika Bhagi-Damodaran
- , Matthew A. Michael
- & Yi Lu
-
Article |
 Chemoproteomic profiling and discovery of protein electrophiles in human cells
Chemoproteomic profiling and discovery of protein electrophiles in human cells
A chemical proteomic strategy is described for the discovery of protein-bound electrophilic groups in human cells. Using this approach, the dynamic regulation of the pyruvoyl catalytic cofactor in S-adenosyl-L-methionine decarboxylase was characterized and an N-terminal glyoxylyl modification on secernin proteins was discovered.
- Megan L. Matthews
- , Lin He
- & Benjamin F. Cravatt
-
Article |
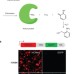 Small-molecule control of protein function through Staudinger reduction
Small-molecule control of protein function through Staudinger reduction
A generally applicable small-molecule switch for protein function in live cells has been developed based on selective protein protection using unnatural amino acid mutagenesis and a bioorthogonal deprotection via Staudinger reduction.
- Ji Luo
- , Qingyang Liu
- & Alexander Deiters
-
Article |
 Installing hydrolytic activity into a completely de novo protein framework
Installing hydrolytic activity into a completely de novo protein framework
Functional catalytic triads have been designed into a hyperstable heptameric α-helical barrel protein. Twenty-one mutations were introduced to form seven Cys-His-Glu catalytic triads. The resulting protein hydrolyses p-nitrophenyl acetate with activities matching the most-efficient redesigned hydrolases based on natural protein scaffolds. This is the first example of a functional catalytic triad being engineered into a fully de novo protein.
- Antony J. Burton
- , Andrew R. Thomson
- & Derek N. Woolfson
-
Article |
 A motif for reversible nitric oxide interactions in metalloenzymes
A motif for reversible nitric oxide interactions in metalloenzymes
NO participates in numerous physiological processes of which many involve the reaction of
NO with metalloenzymes to form a metal–nitrosyl (M–NO). Now, addition ofNO to models of type 1 Cu sites has provided a fully characterized S-nitrosothiol adduct, [CuI](κ1-N(O)SR), that reversibly losesNO upon purging with an inert gas. These findings suggest a new motif for reversible binding of nitric oxide at bioinorganic metal centres.- Shiyu Zhang
- , Marie M. Melzer
- & Timothy H. Warren
-
Article |
 Evolution of thermophilic DNA polymerases for the recognition and amplification of C2ʹ-modified DNA
Evolution of thermophilic DNA polymerases for the recognition and amplification of C2ʹ-modified DNA
Naturally occurring DNA polymerases can amplify DNA efficiently via PCR, but they cannot utilize C2′-modified substrates to make non-natural nucleic acids. Such C2′-modified nucleic acids are of interest as they are resistant to nucleases. Now, a Stoffel fragment DNA polymerase has been evolved to transcribe C2′-modified DNA from a DNA template, reverse transcribe C2′-modified DNA back into DNA, and PCR-amplify C2′-modified DNA.
- Tingjian Chen
- , Narupat Hongdilokkul
- & Floyd E. Romesberg
-
Article |
 Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models
Discovery of a regioselectivity switch in nitrating P450s guided by molecular dynamics simulations and Markov models
A collaborative approach between experiment and simulation has revealed a single mutation in the F/G loop of the newly described nitrating cytochrome P450 TxtE that controls loop dynamics and, more surprisingly, the regioselectivity of the reaction. This mutation is present in a subset of homologous nitrating P450s that produce a previously unidentified biosynthetic intermediate, 5-nitro-L-tryptophan.
- Sheel C. Dodani
- , Gert Kiss
- & Frances H. Arnold
-
Article |
 A simple physical mechanism enables homeostasis in primitive cells
A simple physical mechanism enables homeostasis in primitive cells
The development of cells requires a mechanism to support homeostasis—the maintenance of constant internal conditions—as cellular growth results in internal dilution. Now, a simple physical process is described in which short oligonucleotide inhibitors enable dilution-driven activation of encapsulated ribozymes via membrane growth, suggesting homeostatic mechanisms could have existed in the earliest cells.
- Aaron E. Engelhart
- , Katarzyna P. Adamala
- & Jack W. Szostak
-
Article |
 Reconstitution of [Fe]-hydrogenase using model complexes
Reconstitution of [Fe]-hydrogenase using model complexes
[Fe]-hydrogenase has an iron-guanylylpyridinol cofactor and catalyses the reversible hydrogenation of a methenyl-tetrahydromethanopterin. Now, [Fe]-hydrogenase has been reconstituted using synthetic cofactor mimics. The enzyme containing a mimic with a 2-hydroxy-pyridine group was active, whereas one containing a 2-methoxy-pyridine group was inactive. This result, together with DFT computations, supports a catalytic mechanism involving the deprotonated pyridinol hydroxy group as a proton acceptor.
- Seigo Shima
- , Dafa Chen
- & Xile Hu
-
News & Views |
 Enzyme activity with a twist
Enzyme activity with a twist
A supramolecular polymer comprising stacked artificial chromophores to which zinc(II) complexes are appended is able to respond to enzymatic hydrolysis in aqueous solution. The assembly of molecules can twist reversibly and quickly in response to changes in the type of adenosine phosphate present.
- David B. Amabilino
-
News & Views |
 The power of four
The power of four
Supramolecular assembly has been used to design and create new proteins capable of performing biomimetic functions in complex environments such as membranes and inside living cells.
- Arnold J. Boersma
- & Gerard Roelfes
-
-
-
News & Views |
 Flexibility leads to function
Flexibility leads to function
ATP synthase is an important enzyme for the storage and release of energy in cells. Ion-mobility mass spectrometry has now been used to study its structure, revealing important mechanistic details about its operation and regulation.
- Jianhua Zhao
- & John L. Rubinstein
-
News & Views |
 Cold-hearted RNA heats up life
Cold-hearted RNA heats up life
An RNA replicase ribozyme has long been sought by chemists interested in the origin of life. Now, a selection strategy employing a low-temperature water–ice mixture as the medium has led to discovery of a ribozyme that can catalyse polymerization of an RNA chain greater than its own length.
- Niles Lehman
-
Article |
 In-ice evolution of RNA polymerase ribozyme activity
In-ice evolution of RNA polymerase ribozyme activity
Molecular self-replication through ribozyme-catalysed RNA synthesis could shed light on the origins of life. Here, a polymerase ribozyme capable of synthesizing an RNA sequence longer than itself is described, based on a cold-adapted ribozyme variant evolved in ice. This process demonstrates the potential for the emergence of novel ribozyme phenotypes in altered reaction environments.
- James Attwater
- , Aniela Wochner
- & Philipp Holliger
-
News & Views |
 Proteins in a pill
Proteins in a pill
Protein drugs are important therapies for many different diseases, but very few can be administered orally. Now, a cationic dendronized polymer has been shown to stabilize a therapeutic protein for delivery to the gut.
- Heather D. Maynard
-
Article |
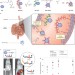 Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates
Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates
Methods for stabilizing enzymatic activity in the gastrointestinal tract are rarely investigated because of the difficulty in protecting proteins from an environment that promotes their digestion. Now, functionally diverse polymers have been conjugated to therapeutic enzymes, which lead to a substantial enhancement of their in vivo activity in the gastrointestinal tract.
- Gregor Fuhrmann
- , Andrea Grotzky
- & Jean-Christophe Leroux
-
Article |
 Studying the role of protein dynamics in an SN2 enzyme reaction using free-energy surfaces and solvent coordinates
Studying the role of protein dynamics in an SN2 enzyme reaction using free-energy surfaces and solvent coordinates
The influence of protein motions on the chemical step of enzyme reactions is a contentious issue. Now, by constructing free-energy surfaces using an explicit solvent coordinate, it is shown that, although some structural flexibility is required, protein motions can be described as equilibrium fluctuations.
- Rafael García-Meseguer
- , Sergio Martí
- & Iñaki Tuñón

 Selection of a promiscuous minimalist cAMP phosphodiesterase from a library of de novo designed proteins
Selection of a promiscuous minimalist cAMP phosphodiesterase from a library of de novo designed proteins On gene silencing by the X10-23 DNAzyme
On gene silencing by the X10-23 DNAzyme Encoding copper catalysts
Encoding copper catalysts