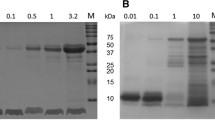
Abstract
Methods to stabilize and retain enzyme activity in the gastrointestinal tract are investigated rarely because of the difficulty of protecting proteins from an environment that has evolved to promote their digestion. Preventing the degradation of enzymes under these conditions, however, is critical for the development of new protein-based oral therapies. Here we show that covalent conjugation to polymers can stabilize orally administered therapeutic enzymes at different locations in the gastrointestinal tract. Architecturally and functionally diverse polymers are used to protect enzymes sterically from inactivation and to promote interactions with mucin on the stomach wall. Using this approach the in vivo activity of enzymes can be sustained for several hours in the stomach and/or in the small intestine. These findings provide new insight and a firm basis for the development of new therapeutic and imaging strategies based on orally administered proteins using a simple and accessible technology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
21 January 2016
In the version of this Article originally published, the concentration of trypsin and chymotrypsin in the Methods section 'In vivo imaging of enzyme activity' should have read "0.4 mg ml-1 in 50 mM phosphate buffer pH 7". This error does not affect the conclusions and has been corrected in the online versions of the Article.
References
Bulow, L. & Mosbach, K. Multienzyme systems obtained by gene fusion. Trends Biotechnol. 9, 226–231 (1991).
Zaks, A. & Klibanov, A. M. Enzymatic catalysis in organic media at 100 °C. Science 224, 1249–1251 (1984).
Klibanov, A. M. Improving enzymes by using them in organic solvents. Nature 409, 241–246 (2001).
Swartz, M. A., Hirosue, S. & Hubbell, J. A. Engineering approaches to immunotherapy. Sci. Transl. Med. 4, 148rv9 (2012).
Lele, B. S., Murata, H., Matyjaszewski, K. & Russell, A. J. Synthesis of uniform protein–polymer conjugates. Biomacromolecules 6, 3380–3387 (2005).
Mahmoud, E. A., Sankaranarayanan, J., Morachis, J. M., Kim, G. & Almutairi, A. Inflammation responsive logic gate nanoparticles for the delivery of proteins. Bioconj. Chem. 22, 1416–1421 (2011).
Zhu, G. Z., Mallery, S. R. & Schwendeman, S. P. Stabilization of proteins encapsulated in injectable poly(lactide-co-glycolide). Nature Biotechnol. 18, 52–57 (2000).
Keefe, A. J. & Jiang, S. Poly(zwitterionic)protein conjugates offer increased stability without sacrificing binding affinity or bioactivity. Nature Chem. 4, 59–63 (2012).
Frokjaer, S. & Otzen, D. E. Protein drug stability: a formulation challenge. Nature Rev. Drug Discov. 4, 298–306 (2005).
Harris, J. M. & Chess, R. B. Effect of PEGylation on pharmaceuticals. Nature Rev. Drug Discov. 2, 214–221 (2003).
Pinier, M., Fuhrmann, G., Verdu, E. & Leroux, J-C. Prevention measures and exploratory pharmacological treatments of celiac disease. Am. J. Gastroenterol. 105, 2551–2561 (2010).
Sarkissian, C. N. et al. A different approach to treatment of phenylketonuria: phenylalanine degradation with recombinant phenylalanine ammonia lyase. Proc. Natl Acad. Sci. USA 96, 2339–2344 (1999).
Enattah, N. S. et al. Identification of a variant associated with adult-type hypolactasia. Nature Genet. 30, 233–237 (2002).
Leeds, J. S., Oppong, K. & Sanders, D. S. The role of fecal elastase-1 in detecting exocrine pancreatic disease. Nature Rev. Gastroenterol. Hepatol. 8, 405–415 (2011).
Fuhrmann, G. & Leroux, J-C. In vivo fluorescence imaging of exogenous enzyme activity in the gastrointestinal tract. Proc. Natl Acad. Sci. USA 108, 9032–9037 (2011).
Shan, L. et al. Structural basis for gluten intolerance in celiac sprue. Science 297, 2275–2279 (2002).
Mitea, C. et al. Efficient degradation of gluten by a prolyl endoprotease in a gastrointestinal model: implications for coeliac disease. Gut 57, 25–32 (2008).
Di Sabatino, A. & Corazza, G. R. Coeliac disease. Lancet 373, 1480–1493 (2009).
Jabri, B. & Sollid, L. M. Tissue-mediated control of immunopathology in coeliac disease. Nature Rev. Immunol. 9, 858–870 (2009).
Tack, G. J., Verbeek, W. H. M., Schreurs, M. W. J. & Mulder, C. J. J. The spectrum of coeliac disease: epidemiology, clinical aspects and treatment. Nature Rev. Gastroenterol. Hepatol. 7, 204–213 (2010).
Sollid, L. M. Coeliac disease: dissecting a complex inflammatory disorder. Nature Rev. Immunol. 2, 647–655 (2002).
Husby, S. et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 54, 136–160 (2012).
Cerf-Bensussan, N., Matysiak-Budnik, T., Cellier, C. & Heyman, M. Oral proteases: a new approach to managing coeliac disease. Gut 56, 157–160 (2007).
Guo, Y. et al. Tuning polymer thickness: synthesis and scaling theory of homologous series of dendronized polymers. J. Am. Chem. Soc. 131, 11841–11854 (2009).
Grotzky, A., Nauser, T., Erdogan, H., Schlüter, A. D. & Walde, P. A fluorescently-labeled dendronized polymer–enzyme conjugate carrying multiple copies of two different types of active enzymes. J. Am. Chem. Soc. 134, 11392–11395 (2012).
Grotzky, A., Manaka, Y., Kojima, T. & Walde, P. Preparation of catalytically active, covalent alpha-polylysine–enzyme conjugates via UV/vis-quantifiable bis-aryl hydrazone bond formation. Biomacromolecules 12, 134–144 (2011).
Shan, L., Mathews, I. I. & Khosla, C. Structural and mechanistic analysis of two prolyl endopeptidases: role of interdomain dynamics in catalysis and specificity. Proc. Natl Acad. Sci. USA 102, 3599–3604 (2005).
Liu, P. & Krishnan, T. R. Alginate-pectin-poly-L-lysine particulate as a potential controlled release formulation. J. Pharm. Pharmacol. 51, 141–149 (1999).
Patel, M. M. et al. Mucin/poly(acrylic acid) interactions: a spectroscopic investigation of mucoadhesion. Biomacromolecules 4, 1184–1190 (2003).
Ward, F. W. & Coates, M. E. Gastrointestinal pH measurement in rats: influence of the microbial flora, diet and fasting. Lab. Anim. 21, 216–222 (1987).
Cook, M. T., Tzortzis, G., Charalampopoulos, D. & Khutoryanskiy, V. V. Microencapsulation of probiotics for gastrointestinal delivery. J. Control. Release 162, 56–67 (2012).
Lehr, C-M., Poelma, F. G. J., Junginger, H. E. & Tukker, J. J. An estimate of turnover time of intestinal mucus gel layer in the rat in situ loop. Int. J. Pharm. 70, 235–240 (1991).
Lai, S. K., Wang, Y-Y. & Hanes, J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61, 158–171 (2009).
Ensign, L. M., Cone, R. & Hanes, J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 64, 557–570 (2012).
Orts Gil, G., Łosik, M., Schlaad, H., Drechsler, M. & Hellweg, T. Properties of pH-responsive mixed aggregates of polystyrene-block-poly(L-lysine) and nonionic surfactant in solution and adsorbed at a solid surface. Langmuir 24, 12823–12828 (2008).
Nilsson, F. & Johansson, H. A double isotope technique for the evaluation of drug action on gastric evacuation and small bowel propulsion studied in the rat. Gut 14, 475–477 (1973).
Torjman, M. C., Joseph, J. I., Munsick, C., Morishita, M. & Grunwald, Z. Effects of isoflurane on gastrointestinal motility after brief exposure in rats. Int. J. Pharm. 294, 65–71 (2005).
Green, P. H. R. & Cellier, C. Celiac disease. New Engl. J. Med. 357, 1731–1743 (2007).
Turner, J. R. Intestinal mucosal barrier function in health and disease. Nature Rev. Immunol. 9, 799–809 (2009).
Blau, N., van Spronsen, F. J. & Levy, H. L. Phenylketonuria. Lancet 376, 1417–1427 (2010).
Sarkissian, C. N., Kang, T. S., Gamez, A., Scriver, C. R. & Stevens, R. C. Evaluation of orally administered PEGylated phenylalanine ammonia lyase in mice for the treatment of phenylketonuria. Mol. Genet. Metab. 104, 249–254 (2011).
Griffiths, P. C. et al. PGSE-NMR and SANS studies of the interaction of model polymer therapeutics with mucin. Biomacromolecules 11, 120–125 (2009).
Vandamme, T. F. & Brobeck, L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J. Control. Release 102, 23–38 (2005).
DiMagno, E. P., Go, V. L. W. & Summerskill, W. H. J. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. New Engl. J. Med. 288, 813–815 (1973).
Domínguez–Muñoz, J. E. Chronic pancreatitis and persistent steatorrhea: what is the correct dose of enzymes? Clin. Gastroenterol. Hepatol. 9, 541–546 (2011).
Fieker, A., Philpott, J. & Armand, M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin. Exp. Gastroenterol. 4, 55–73 (2011).
Hawker, C. J. & Wooley, K. L. The convergence of synthetic organic and polymer chemistries. Science 309, 1200–1205 (2005).
Matyjaszewski, K. & Tsarevsky, N. V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nature Chem. 1, 276–288 (2009).
Gauthier, M. A. & Klok, H-A. Polymer–protein conjugates: an enzymatic activity perspective. Polym. Chem. 1, 1352–1373 (2010).
Bertrand, N. & Leroux, J-C. The journey of a drug-carrier in the body: an anatomo-physiological perspective. J. Control. Release 161, 152–163 (2012).
Acknowledgements
We thank B. Castagner, K. Fuhrmann, S. T. Proulx and J. Scholl for their technical help. Financial support from the Swiss National Science Foundation (310030_135732) and IG Zöliakie der Deutschen Schweiz is acknowledged.
Author information
Authors and Affiliations
Contributions
G.F., M.A.G. and J-C.L. designed and conceived the study; PW and ADS participated in the design of the dendronized polymers and coupling chemistry; G.F. and A.G. prepared and characterized conjugates in vitro with the help of R.L. and S.M.; G.F. conducted and analysed all in vivo experiments; P.L., B.Z. and H.Y. synthesized and contributed compounds; G.F., M.A.G., A.G., P.W., A.D.S. and J-C.L. co-wrote the paper. All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 6053 kb)
Rights and permissions
About this article
Cite this article
Fuhrmann, G., Grotzky, A., Lukić, R. et al. Sustained gastrointestinal activity of dendronized polymer–enzyme conjugates. Nature Chem 5, 582–589 (2013). https://doi.org/10.1038/nchem.1675
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1675
This article is cited by
-
Stimuli-responsive rotaxane-branched dendronized polymers with tunable thermal and rheological properties
Nature Communications (2023)
-
Protein transfection via spherical nucleic acids
Nature Protocols (2022)
-
Nanocomposite systems for precise oral delivery of drugs and biologics
Drug Delivery and Translational Research (2021)
-
Thermoresponsive Nanogels from Dendronized Copolymers for Complexation, Protection and Release of Nucleic Acids
Chinese Journal of Polymer Science (2020)
-
Non-canonical amino acid labeling in proteomics and biotechnology
Journal of Biological Engineering (2019)