Abstract
CD68 plays a critical role in promoting phagocytosis; however, the function of CD68 in tumor immunity and prognosis remains unknown. We analyzed CD68 expression among 33 tumor and normal tissues from The Cancer Genome Atlas and Genotype-Tissue Expression datasets. The relationship between CD68 expression and cancer prognosis, immune infiltration, checkpoint markers, and drug response was explored. Upregulated CD68 levels were observed in various cancer types, which were verified through tumor tissue chips using immunohistochemistry. High levels of CD68 in tumor samples correlated with an adverse prognosis in glioblastoma, kidney renal clear cell carcinoma, lower-grade glioma, liver hepatocellular carcinoma, lung squamous cell carcinoma, thyroid carcinoma, and thymoma and a favorable prognosis in kidney chromophobe. The top three negatively enriched Kyoto Encyclopedia of Genes and Genomes terms in the high CD68 subgroup were chemokine signaling pathway, cytokine-cytokine receptor interaction, and cell adhesion molecule cams. The top negatively enriched HALLMARK terms included complement, allograft rejection, and inflammatory response. A series of targeted drugs and small-molecule drugs with promising therapeutic effects were predicted. The clinical prognosis and immune infiltration of high expression levels of CD68 differ across tumor types. Inhibiting CD68-dependent signaling could be a promising therapeutic strategy for immunotherapy in many tumor types.
Similar content being viewed by others
Introduction
According to a recent study in 2019, cancer has become the leading or second most common cause of death in more than 112 countries in people less aged than 70 years1. Worldwide, more than 19 million new cancer cases and 10 million cancer-related deaths occurred in 20202. Despite undergoing a series of traditional treatment methods, including radiotherapy, chemotherapy, biological therapy, and surgery, the effect of tumor therapy remains unsatisfactory3,4. Tumor immunotherapy strategies, such as focusing on programmed cell death protein 1 (PD-1), were shown to be novel and promising treatments for tumors5,6. With the rapid development of high-throughput sequencing technology, an increasing number of immune-associated molecules related to tumor prognosis have been discovered, which may play an irreplaceable role in tumor immunotherapy.
The cluster of differentiation 68 (CD68), also known as GP110, LAMP4, or SCARD1, is a 110 kDa transmembrane glycoprotein that is widely expressed in monocyte cell types, such as macrophages, microglia, and osteoclasts7. CD68 plays an essential role in various physiological and pathological processes, including atherosclerosis formation8, inflammation and auto-immunity9, bone-resorbing promotion10, and tumor progression11,12. Bone marrow-derived macrophages are the most common type of tumor-infiltrating immune cells in the tumor microenvironment (TME) and are vital mediators of the antitumor immune response13,14,15. Recent studies have found that CD68 is overexpressed in tumor-associated macrophages (TAMs) and tumor cells. High levels of CD68 are associated with a higher tumor grade, larger tumor size, Ki67 positivity, and other malignant features, which indicate tumor progression and aggressiveness16,17,18,19. TAMS, identified by CD68 expression, can be divided into two subtypes: classically activated type 1(M1-like) macrophages and alternatively activated type 2 (M2-like) macrophages. M1-like macrophages, with proinflammatory characteristics that express high levels of free radicals and major histocompatibility complex molecules, contribute to antitumor activity20,21. In contrast, M2-like macrophages, which release multiple anti-inflammatory cytokines and chemokines, promote tumor growth and metastasis22,23. Increasing evidence has shown that CD68 is a promising tumor-associated diagnostic and prognostic marker for cancer. However, the signaling pathways in which CD68 is involved in tumor immunity and progression remain unclear.
In this study, we studied the expression of CD68 in 33 cancer types using large-scale RNA-sequencing (RNA-seq) data from The Cancer Genome Atlas (TCGA). Upregulated levels of CD68 were observed in various cancer types, which were observed in the TCGA database and our tumor tissue chips. We also discussed the value of CD68 in the prognostic prediction of pan-cancer. Moreover, the relationship between the expression levels of CD68 and the infiltration of immune cells in the pan-cancer microenvironment was observed. Finally, we analyzed the correlation between a series of predicted drugs and CD68 expression, which may be used for tumor immunotherapy in the future.
Methods
Collection of sample and patient data
The clinicopathological features and RNA-sequencing (RNA-seq) data of the 33 types of cancer were chosen from the TCGA dataset (http://cancergenome.nih.gov). As the data from normal tissue is relatively insufficient, the RNA-seq data of normal human tissues were additionally added from the GTEx dataset (https://www.gtexportal.org/) to analyze the expression levels of CD68 between tumor and normal tissues. A tumor tissue chip (catalog No. BCN963) contains multiple organ tumor arrays with matched normal tissues that were used to verify the expression of CD68. Informed consent was obtained from all participants in this study.
Recognition of relevant features
The gene expression data of CD68 were extracted from the TCGA and GTEx databases to form an expression matrix using GEPIA (http://gepia.cancer-pku.cn/) and R package (4.0.4). The genetic mutation aspects of CD68 were obtained from the public database CBIOPORTAL (https://www.cbioportal.org/). A Kaplan–Meier (KM) analysis with a log-rank test was used to compare the disease-free interval (DFI), progression-free interval (PFI), disease-specific survival (DSS), and overall survival (OS) of the patients. In this study, we selected the optimal cutoff point to create KM curves based on the coxph function of R package survival. A univariate Cox model was used to calculate the relationship between CD68 expression levels and patient survival. The immune infiltrates among 33 types of cancers were studied using the Tumor Immune Estimation Resource (TIMER 2.0, https://cistrome.shinyapps.io/timer/)24 and CIBERSORT25. The ESTIMATE algorithm was applied to estimate the stromal and immune cells in the tumor microenvironment and to calculate the stromal and immune scores and estimate scores. Gene set enrichment analysis (GSEA) was used to display the biological functions and pathways involved in CD68. This analysis was implemented in Sangerbox (http://sangerbox.com/) based on the Molecular Signatures Database (MSigDB) H (hallmark gene sets) and Kyoto Encyclopedia of Genes and Genomes database (KEGG)26,27,28. The relationship between CD68 expression and drug responses was predicted using CELLMINER (http://discover.nci.nih.gov/cellminer/) using the R language. In addition, sensitive drugs based on CD68 levels were analyzed from the Clinical Trials Reporting Program and Genomics of Drug Sensitivity in Cancer database.
Immunohistochemistry
In this study, the immunohistochemistry protocol was used to stain the tissue chip BCN963 (Bioyeargene Biosciences company, Wuhan, China) as previously reported29. Briefly, sections were obtained from formalin-fixed, paraffin-embedded tissues of normal and pan-cancer. After antigen retrieval and blocking of endogenous HRP activity, the slides were blocked with 10% normal goat serum and incubated with a primary antibody at 4 °C overnight. Then, the secondary antibody was added and incubated at room temperature for 50 min. Slides were counterstained with hematoxylin, and representative images were obtained using an Olympus inverted microscope. The primary antibodies used were polyclonal rat anti-CD68 (AF20022, AiFang Biological, China, 1:200 dilution). HRP-labeled goat anti-rabbit IgG (GB23303, ServiceBio, 1:200 dilution) was used as the secondary antibody. We optimized the final concentration through a pre-experiment and the protocol of the antibody. Negative control procedures included the omission of the primary antibody.
Statistical analysis
A student’s t-test was performed to explore the correlation between CD68 expression and the drugs. The Kruskal–Wallis test was used to compare the expression levels of CD68 in tumor and normal tissues. KM curves, the log-rank test, and the Cox proportional hazards regression model were applied to analyze the survival conditions. In addition, Spearman’s test was used for the correlation analysis. All analyses were performed using the R language. All statistical tests were two-sided, and statistical significance was set at a P-value of < 0.05.
Ethical approval and ethical standards
The study with primary human tissues was approved by the ethics committee of the Xiangya Hospital, Central South University, and the procedures with human samples were performed in accordance with the ethical standards of the ethics committee and the Helsinki Declaration of 1975 and its later amendments.
Results
Expression of CD68 in pan-cancer
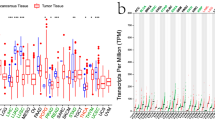
First, to fully clarify the expression of CD68 in pan-cancer, we matched the GTEx normal samples with TCGA tumor samples (Fig. 1A). We found that the levels of CD68 were significantly elevated (P < 0.01) in colon adenocarcinoma (COAD), glioblastoma multiforme (GBM), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), brain low-grade glioma (LGG), ovarian serous cystadenocarcinoma (OV), pancreatic adenocarcinoma (PAAD), rectum adenocarcinoma (READ), skin cutaneous melanoma (SKCM), stomach adenocarcinoma (STAD), testicular germ cell tumors (TGCT), and uterine carcinosarcoma (UCS) compared to in normal tissues. In contrast, CD68 was significantly decreased (P < 0.001) in thymoma (THYM) compared with GTEx normal controls. Moreover, we observed the expression of CD68 in these tumor and normal controls using tissue chip. Each tumor/normal tissue has three cores (diameter 1 mm). Even though statistical analysis was not possible due to the small sample size, we compared the expression of CD68 in these tumor tissues and normal tissues and found that the immunohistoactivity of CD68 was obviously enhanced in COAD, GBM, KIRC, LGG, OV, PAAD, READ, SKCM, and STAD compared with their normal controls (Fig. 1B), which might provide evidence for the difference in CD68 gene expression in Fig. 1A.
Expresssion aspect of CD68 in tumor and normal tissues based on the TCGA and GETx databases. CD68 expression from the TCGA and GTEx databases (A). CD68 expression from tumor tissue chip using immunohistochemistry, n = 3 for tumor/control groups in COAD, GBM, KIRC, LGG, OV, PAAD, READ, SKCM, and STAD (B). *P < 0.05, **P < 0.01, ***P < 0.001.
Mutation profile and prognostic value of CD68 in pan-cancer
We then checked the mutant landscape of CD68 in different cancer types from the TCGA database using cBioportal (Fig. 2). The data showed that prostate adenocarcinoma (PRAD) and diffuse large B-cell lymphoma had a high mutation level with CD68 deep deletion of more than 4% (Fig. 2A,B). A total of 45 mutation sites (including 31 missense, 11 truncating, two splices, and one inflame) were found between amino acids 0 and 354 (Fig. 2C). Next, to understand the prognostic value of CD68 in pan-cancer further, we downloaded RNA-seq and clinical data of CD68 from the TCGA dataset. Elevated levels of CD68 were significantly related to poorer OS in GBM (hazard ratio [HR] 1.05, 95% confidence interval [CI] 1.00–1.09, P = 0.0370), KIRC (HR 1.04, 95% CI 1.0–21.06, P = 0.0007), LGG (HR 1.18, 95% CI 1.06–1.31, P = 0.0020), liver hepatocellular carcinoma (LIHC) (HR 1.06, 95% CI 1.02–1.11, P = 0.0058), lung squamous cell carcinoma (LUSC) (HR 1.04, 95% CI 1.00–1.08, P = 0.0470), thyroid carcinoma (THCA) (HR 1.17, 95% CI 1.06–1.29, P = 0.0023), and thymoma (THYM) (HR 1.47, 95% CI 1.08–2.00, P = 0.0130) (Fig. 3). In contrast, upregulated CD68 expression was associated with a favorable prognosis in kidney chromophobe (KICH) (HR 1.40, 95% CI 1.05–1.87, P = 0.0220). In addition, the high expression of CD68 was related to an unfavorable DSS in KIRC (HR 1.04, 95% CI 1.02–1.07, P = 0.0052), GBM (HR 1.05, 95% CI 1.00–1.10, P = 0.0490), LGG (HR 1.19, 95% CI 1.06–1.34, P = 0.0410), and THYM (HR 1.69, 95% CI 1.04–2.77, P = 0.0360) (Fig. 3B and Supplementary Fig. 1C–I). However, DSS was favorable in cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC) (HR 0.83, 95% CI 0.70–0.99, P = 0.0380) and KICH (HR 1.46, 95% CI 1.09–1.96, P = 0.0110). Furthermore, we observed the prognostic value of CD68 in DFI (Supplementary Fig. 1A) and PFI (Supplementary Fig. 1B). The results showed that high levels of CD68 were associated with a poorer DFI in GBM, LGG, and THYM and a better DFI in KICH (Supplementary Fig. 1E–I). High levels of CD68 were associated with a poorer PFI in cholangiocarcinoma (CHOL), LIHC, and STAD and a better DFI in CESC (Supplementary Fig. 1J–M).
Survival analysis of CD68 in pan-cancer from the TCGA database. Forest plot compared the prognostic value of CD68 on OS (A) and DSS (B) across pan-cancer. Kaplan–Meier Method displayed the prognostic value of CD68 on OS in GBM (C), KICH (D), KIRC (E), LGG (F), LIHC (G), LUSC (H), THCA (I), THYM (J). The cut-off points are 61.89% (GBM), 73.44% (KICH), 15.66% (KIRC), 20.04% (LGG), 15.14% (LIHC), 16.57% (LUSC), 10.96% (THCA), 16.95% (THYM). *P < 0.05, **P < 0.01, ***P < 0.001.
Relationship between CD68 expression and immune infiltrates
Next, we explored the relationship between CD68 expression and immune infiltrates in the tumor microenvironment in 33 tumor types based on the TIMER2.0 database. As shown in Fig. 4, CD68 expression was positively correlated with immune cell infiltration, including dendritic cells, monocytes, macrophages, and neutrophils. However, CD68 expression is negatively associated with the infiltration of myeloid-derived suppressor cells. Next, we analyzed the correlation between CD68 levels and immune cell infiltration in the tumor microenvironment in 33 cancer types. The results indicated that the expression of CD68 was positively related to the abundance of B cells, CD4+ and CD8+ T cells, dendritic cells, macrophages, and neutrophils in many tumor types. As shown in Fig. 5A, the three most significantly related tumors were adrenocortical carcinoma (ACC), BRCA, and CESC. The details of other tumor types are shown in Supplementary Fig. 2. We calculated the stromal, immune, and estimated score of 33 cancer types using the ESTIMATE algorithm. As shown in Fig. 5B, the top three tumor types with CD68 expression positively correlated with stromal score were BLCA, BRCA, and GBM (P < 0.001). The top three tumor types with CD68 expression positively correlated with immune score were ACC, BLCA, and BRCA (p < 0.001). The top three tumor types with CD68 expression positively correlated with the estimate score were BLCA, BRCA, and CESC (P < 0.001). The data in Supplementary Fig. 3 shows that the expression of CD68 was significantly and positively correlated with the stromal score in all tumor types except CHOL and mesothelioma (MESO). In addition, CD68 levels were found to significantly and positively correlate with the immune score (Supplementary Fig. 4) and estimate the score (Supplementary Fig. 5) in all tumor types. These results indicate that CD68 has a close relationship with immune infiltrates in the tumor microenvironment and might act as a promising immunotherapy target.
The relationship between CD68 and immune infiltrates based on the TIMER2.0 analysis in pan-cancer. The correlation between CD68 with B cell, CD4+ T cell, CD8+ T cell, dendritic cell, monocyte, macrophage in pan-cancer (A). The correlation between CD68 with NK cell, neutrophil, Tregs, mast cell, NKT, Tfh, γδT, HSC, Endo, progenitor, Eos, CAF, and MDSC in pan-cancer (B).
Tumor neoantigens are foreign proteins absent from normal human organs/tissues and are encoded by a mutated gene of tumor cells, which plays a crucial role in tumor immunotherapy. We then explored the relationship between CD68 expression and the number of neoantigens in human cancers (Fig. 6 and Supplementary Table 1). Our results indicated that high levels of CD68 were significantly and positively related to the number of neoantigens in LUAD, KIRP, CESC, and PRAD (P < 0.05).
Relationship between CD68 expression and checkpoint gene markers, tumor mutation burden, and microsatellite instability
To further elucidate the potential immune mechanisms of CD68, we next compared the association of CD68 expression with various checkpoint markers in different cancer types (Fig. 7A). The results showed that CD68 expression positively correlated with the expression of LAIR1, HAVCR2, LGALS9, and PD-1 (PDCD1) in most of the 33 tumor types. We also studied the relationship between CD68 expression and five DNA mismatch repair (MMR) markers (Fig. 7B). CD68 levels were significantly and negatively correlated with mutL homolog 1, mutS homolog 2, mutS homolog 6 (MSH6), postmeiotic segregation increased 2 (PMS2), and epithelial cell adhesion molecule in BRCA, CESC, KIRC, OV, and THCA (P < 0.05). However, CD68 levels were significantly and positively correlated with MSH6 in KICH and READ (P < 0.05). In addition, we studied the correlation between tumor mutation burden (TMB) and microsatellite instability (MSI) with CD68 levels. Moreover, CD68 expression was positively correlated (P < 0.05) with TMB in UCEC, SKCM, sarcoma (SARC), READ, PRAD, LGG, KIRP, KIRC, COAD, CESC, and BRCA, and negatively correlated (P < 0.05) with TMB in THCA, READ, LIHC, LAML, and GBM (Fig. 7C). CD68 expression was positively correlated (P < 0.05) with MSI in UCEC, READ, LIHC, and COAD, but negatively correlated (P < 0.05) with MSI in KIRC, LUAD, LUSC, and TGCT (Fig. 7D). In addition, we analyzed the prognostic value of the combination of CD68 expression and these markers (MMR markers and PD-1). The results indicated that the combination of CD68 expression and MMR markers had prognostic value in KIRC, LGG, PAAD, and SARC (Supplementary Fig. 6A). The combination of CD68 expression and PD-1 had prognostic value in KIRC, LGG, SKCM, and THYM (Supplementary Fig. 6B).
Functional analysis by GSEA and drug response of CD68
In addition, we analyzed the related functional signaling pathways of CD68 through GSEA based on KEGG and HALLMARK databases in pan-cancer. The top three negatively enriched KEGG terms (P < 0.001) in the upregulated CD68 subgroup were the chemokine signaling pathway, cytokine-cytokine receptor interaction, and cell adhesion molecule cams (Fig. 8A). The top three positively enriched KEGG terms in the upregulated CD68 subgroup were aminoacyl tRNA biosynthesis, valine leucine, and isoleucine biosynthesis and taste transduction (Fig. 8B). In addition, the top three negatively enriched HALLMARK terms (P < 0.001) in the upregulated CD68 subgroup were a complement, allograft rejection, and inflammatory response (Fig. 8C). The top three positively enriched HALLMARK terms in the upregulated CD68 subgroup were pancreatic beta cells and MYC targets V1 and V2 (Fig. 8D). The top five enriched pathways are shown in Supplementary Table 2.
Finally, we analyzed three public databases (CellMiner, CTRP, and Genomics of Drug Sensitivity in Cancer [GDSC]) to identify small molecules and sensitive drugs based on CD68 expression. The results from drug response analysis by CellMiner suggested that many small molecules were associated with CD68 expression, of which the top 16 are shown in Supplementary Fig. 7. These small molecules were found to be active in inhibiting human tumor cell line growth, including adenocarcinoma, non-small lung, melanoma, prostate, CNS, and colon. In addition, the top 20 sensitive drugs predicted from the CTRP (Supplementary Table 3) and GDSC (Supplementary Table 4) databases exhibited a close relationship with the metabolism and activation of macrophages.
Discussion
With the rapid development of high-throughput sequencing in recent years, various immune markers in TME that are related to tumor progression have been identified, providing new directions for tumor therapy30,31. In this study, we explored the role of CD68 in clinical outcome prediction and immune cell infiltration in pan-cancer from the TCGA and GETx databases. The results indicated that elevated CD68 was observed in many tumor types, including COAD, GBM, KIRC, KIRP, LGG, OV, PAAD, READ, SKCM, STAD, TGCT, and UCS, and was associated with a more unsatisfactory clinical outcome in GBM, KIRC, LGG, LIHC, LUSC, THCA, and THYM. We also calculated the infiltration of immune cells in the tumor microenvironment. As an important marker of macrophages, CD68 is expressed not only in macrophages, but also in other immune cells and certain tumors32. We found that high levels of CD68 were associated with many immune cells in the tumor microenvironment, such as monocytes, abundant B cells, CD4+ and CD8+ T cells, dendritic cells, macrophages, and neutrophils. Moreover, the upregulated expression of CD68 was closely related to the stromal, immune, and estimated scores in many types of human cancers. These results were consistent with previous studies in CD6833,34,35, which indicated that CD68 might be a novel and promising immunotherapy target in the future.
Neoantigens, which are nonautologous proteins with specific characteristics, are generated from the tumor cell genome through nonsynonymous mutations36 and play an essential role in tumor immunotherapy36,37,38. The present study illustrated that high expression levels of CD68 were significantly and positively related to the number of neoantigens in LUAD, KIRP, CESC, and PRAD. In addition, the mutation landscape of CD68 in pan-tumor types was also observed. Anti-immune checkpoint therapy has become a necessary treatment for cancer in recent years36,39,40. To fully clarify the immune value of CD68 in pan-cancer, we next studied the correlation between CD68 expression and large numbers of immune checkpoints in pan-cancer and found that high levels of CD68 were significantly and positively correlated with some key checkpoints, including LAIR1, HAVCR2, LGALS9, and PD-1 in most of the 33 tumor types. Furthermore, in this study, CD68 expression was found to be negatively correlated with DNA mismatch repair (MMR) markers in most types of cancer. Increasing studies have found that TMB and MSI are emerging clinical biomarkers in immunotherapy, clinical outcomes, and chemotherapy sensitivity in various tumor types41,42,43. The present study also found that CD68 expression was correlated with TMB and MSI in many cancer types, which might provide probable and potential evidence for predicting the efficacy of tumor immunotherapy. The specific mechanisms of CD68 in tumor growth and metastasis are unclear. Our study explored the majority of pathways related to the expression of CD68 in pan-cancer, which might help determine the exact function of CD68 and downstream signaling pathways in the future.
After experiencing many disappointing results in recent years, tumor immunotherapy that focuses on inhibiting immune checkpoint pathways has become a clinically promising treatment for many cancers44,45. For example, the PD-1/PD-L1 signaling pathway plays a vital role in the inhibition of immune responses and induction of apoptosis. Several studies have indicated that specific antibodies or drugs that target the PD-1/PD-L1 axis can effectively promote antitumor immunotherapy and lead to a better prognosis in several cancer types46,47,48. Cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), expressed by activated effector T cells, is an important regulator that participates in T cell proliferation and cytokine production49. Immunotherapies targeting the CTLA-4 pathway have shown remarkable clinical efficacy against many cancer types, such as prostate, cervical, gastric, pancreatic, ovarian, and urothelial cancers and melanoma50,51. In this study, we found that CD68 is closely related to tumor immunity in the TME and may act as a new immune checkpoint in tumor treatments in the future. To the best of our knowledge, there are no small-molecule drugs that specifically target CD68 in tumor therapy. Finally, we identified a series of targeted medicines and small-molecule drugs with promising efficacy predicted by CD68 levels, as proven by the Food and Drug Administration. These sensitive drugs are closely correlated with the proliferation and activation of macrophages. For example, cerulenin, ciclopirox, and PRIMA-1 were the top three predicted drugs that were positively related to CD68 expression based on the CTRP database. Fatty acid synthase, which induces the synthesis of long-chain fatty acids, prevents proinflammatory progress in macrophages. Cerulenin was found to play a vital role in inhibiting FA synthases in macrophages52. Ciclopirox, an iron chelator, can regulate macrophage phagocytosis by depriving iron from macrophages53. PRIMA-1, a mutant p53 reactivator, regulates the polarization of macrophages by inhibiting the NF-κB and STAT1 signaling pathways54. Moreover, trametinib, RDEA119, and selumetinib were the top three predicted drugs that were negatively related to CD68 expression based on the GDSC database. Trametinib, RDEA119, and selumetinib, which are effective MEK kinase inhibitors, can significantly reduce the proliferation and migration of macrophages55,56. These drugs might play a key role in tumor chemotherapy and are conducive to improving the treatment of tumors.
This study had several limitations. First, the messenger ribonucleic acid expression levels of CD68 were assessed from public databases and only verified by tumor tissue chips, not validated by in vivo and in vitro studies. Second, the role of CD68 in tumor immune cell infiltration in pan-cancer was not verified by cell and animal experiments in this study. More studies focusing on the specific signaling pathways of CD68 in pan-cancer need to be conducted in the future.
In summary, in this paper, we systematically discussed the aspects related to expression and mutant characteristics of CD68 in pan-cancer. CD68 levels were found to be elevated in many cancer types and showed significant prognostic value. These results, together with previous studies, demonstrate that CD68 in the TME is a very promising biomarker in these tumors. In addition, CD68 was found to be closely correlated with immune infiltrates in the TME and could a promising immunotherapy target that positively correlates with other checkpoint proteins in pan-cancer. Specific antibodies or inhibitors that neutralizing CD68 in the TME in there cancers might provide a new direction for tumor immunotherapy, especially targeting these immune cells that significantly associated with CD68 expression, such as B cells, CD4+ and CD8+ T cells, dendritic cells, macrophages, and neutrophils. Moreover, we explored various small molecules and predicted drugs that target CD68 in pan-cancer. Multicenter clinical trials that focus on the efficacy, safety, or risk benefit ratio of small molecules and predicted drugs might benefit patients with elevated CD68 in these cancer tissues. Therefore, further research is needed to explore the therapeutic effects of these molecules for pan-cancer treatment through basic and clinical experiments.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. https://doi.org/10.3322/caac.21660 (2021).
Malapelle, U., Muscarella, L. A., Pisapia, P. & Rossi, A. Targeting emerging molecular alterations in the treatment of non-small cell lung cancer: Current challenges and the way forward. Expert Opin. Investig. Drugs 29, 363–372. https://doi.org/10.1080/13543784.2020.1732922 (2020).
Zugazagoitia, J. et al. Current challenges in cancer treatment. Clin. Ther. 38, 1551–1566. https://doi.org/10.1016/j.clinthera.2016.03.026 (2016).
Farkona, S., Diamandis, E. P. & Blasutig, I. M. Cancer immunotherapy: The beginning of the end of cancer?. BMC Med. 14, 73. https://doi.org/10.1186/s12916-016-0623-5 (2016).
Youlin, K., Li, Z., Xiaodong, W., Xiuheng, L. & Hengchen, Z. Combination immunotherapy with 4–1BBL and CTLA-4 blockade for the treatment of prostate cancer. Clin. Dev. Immunol. 2012, 439235. https://doi.org/10.1155/2012/439235 (2012).
Holness, C. L. & Simmons, D. L. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood 81, 1607–1613 (1993).
de Beer, M. C., Zhao, Z., Webb, N. R., van der Westhuyzen, D. R. & de Villiers, W. J. Lack of a direct role for macrosialin in oxidized LDL metabolism. J. Lipid Res. 44, 674–685. https://doi.org/10.1194/jlr.M200444-JLR200 (2003).
Song, L., Lee, C. & Schindler, C. Deletion of the murine scavenger receptor CD68. J. Lipid Res. 52, 1542–1550. https://doi.org/10.1194/jlr.M015412 (2011).
Ashley, J. W. et al. Genetic ablation of CD68 results in mice with increased bone and dysfunctional osteoclasts. PLoS ONE 6, e25838. https://doi.org/10.1371/journal.pone.0025838 (2011).
Steinert, G. et al. Immune escape and survival mechanisms in circulating tumor cells of colorectal cancer. Cancer Res. 74, 1694–1704. https://doi.org/10.1158/0008-5472.CAN-13-1885 (2014).
Troiano, G. et al. Prognostic significance of CD68(+) and CD163(+) tumor associated macrophages in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral. Oncol. 93, 66–75. https://doi.org/10.1016/j.oraloncology.2019.04.019 (2019).
De Palma, M. & Lewis, C. E. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell 23, 277–286. https://doi.org/10.1016/j.ccr.2013.02.013 (2013).
Vitale, I., Manic, G., Coussens, L. M., Kroemer, G. & Galluzzi, L. Macrophages and metabolism in the tumor microenvironment. Cell. Metab. 30, 36–50. https://doi.org/10.1016/j.cmet.2019.06.001 (2019).
Zhu, J. et al. The role of tumor associated macrophages in the tumor microenvironment: Mechanism and functions. Anticancer Agents Med. Chem. 16, 1133–1141. https://doi.org/10.2174/1871520616666160520112622 (2016).
Mazur, G., Halon, A., Wrobel, T. & Kuliczkowski, K. Macrophage/histiocytic antigen CD68 expression in neoplastic and reactive lymph nodes. Rocz. Akad. Med. Bialymst 49(Suppl 1), 73–75 (2004).
Medrek, C., Ponten, F., Jirstrom, K. & Leandersson, K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer 12, 306. https://doi.org/10.1186/1471-2407-12-306 (2012).
Ni, C. et al. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: A retrospective study and meta-analysis. J. Cancer 10, 4463–4472. https://doi.org/10.7150/jca.33914 (2019).
Sun, S. et al. The expression and relationship of CD68-tumor-associated macrophages and microvascular density with the prognosis of patients with laryngeal squamous cell carcinoma. Clin. Exp. Otorhinolaryngol. 9, 270–277. https://doi.org/10.21053/ceo.2015.01305 (2016).
Maccio, A. et al. Role of M1-polarized tumor-associated macrophages in the prognosis of advanced ovarian cancer patients. Sci. Rep. 10, 6096. https://doi.org/10.1038/s41598-020-63276-1 (2020).
Murray, P. J. & Wynn, T. A. Obstacles and opportunities for understanding macrophage polarization. J. Leukoc. Biol. 89, 557–563. https://doi.org/10.1189/jlb.0710409 (2011).
Sumitomo, R. et al. M2 tumor-associated macrophages promote tumor progression in non-small-cell lung cancer. Exp. Ther. Med. 18, 4490–4498. https://doi.org/10.3892/etm.2019.8068 (2019).
Sumitomo, R. et al. PD-L1 expression on tumor-infiltrating immune cells is highly associated with M2 TAM and aggressive malignant potential in patients with resected non-small cell lung cancer. Lung Cancer 136, 136–144. https://doi.org/10.1016/j.lungcan.2019.08.023 (2019).
Li, T. et al. TIMER: A web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Res. 77, e108–e110. https://doi.org/10.1158/0008-5472.CAN-17-0307 (2017).
Newman, A. M. et al. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat. Biotechnol. 37, 773–782. https://doi.org/10.1038/s41587-019-0114-2 (2019).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Ishiguro-Watanabe, M. & Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucleic Acids Res. 49, D545–D551. https://doi.org/10.1093/nar/gkaa970 (2021).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Zhang, H. et al. PDIA5 is correlated with immune infiltration and predicts poor prognosis in gliomas. Front. Immunol. 12, 628966. https://doi.org/10.3389/fimmu.2021.628966 (2021).
Liu, F. et al. CD96, a new immune checkpoint, correlates with immune profile and clinical outcome of glioma. Sci. Rep. 10, 10768. https://doi.org/10.1038/s41598-020-66806-z (2020).
Liu, F. et al. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell. Int. 20, 7. https://doi.org/10.1186/s12935-019-1085-6 (2020).
Ruffell, B. & Coussens, L. M. Macrophages and therapeutic resistance in cancer. Cancer Cell 27, 462–472. https://doi.org/10.1016/j.ccell.2015.02.015 (2015).
Ceci, C., Atzori, M. G., Lacal, P. M. & Graziani, G. Targeting tumor-associated macrophages to increase the efficacy of immune checkpoint inhibitors: A glimpse into novel therapeutic approaches for metastatic melanoma. Cancers https://doi.org/10.3390/cancers12113401 (2020).
Makitie, T., Summanen, P., Tarkkanen, A. & Kivela, T. Tumor-infiltrating macrophages (CD68(+) cells) and prognosis in malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 42, 1414–1421 (2001).
Strojnik, T. et al. Prognostic impact of CD68 and kallikrein 6 in human glioma. Anticancer Res. 29, 3269–3279 (2009).
Srivastava, P. K. Neoepitopes of cancers: Looking back looking ahead. Cancer Immunol. Res. 3, 969–977. https://doi.org/10.1158/2326-6066.CIR-15-0134 (2015).
Jiang, T. et al. Tumor neoantigens: From basic research to clinical applications. J. Hematol. Oncol. 12, 93. https://doi.org/10.1186/s13045-019-0787-5 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74. https://doi.org/10.1126/science.aaa4971 (2015).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 161, 205–214. https://doi.org/10.1016/j.cell.2015.03.030 (2015).
Toor, S. M., Sasidharan Nair, V., Decock, J. & Elkord, E. Immune checkpoints in the tumor microenvironment. Semin. Cancer Biol. 65, 1–12. https://doi.org/10.1016/j.semcancer.2019.06.021 (2020).
Li, K., Luo, H., Huang, L., Luo, H. & Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell. Int. 20, 16. https://doi.org/10.1186/s12935-019-1091-8 (2020).
Nojadeh, J. N., Behrouz Sharif, S. & Sakhinia, E. Microsatellite instability in colorectal cancer. EXCLI J. 17, 159–168. https://doi.org/10.17179/excli2017-948 (2018).
Shao, C. et al. Prevalence of high tumor mutational burden and association with survival in patients with less common solid tumors. JAMA Netw. Open 3, e2025109. https://doi.org/10.1001/jamanetworkopen.2020.25109 (2020).
Durant, J. R. Immunotherapy of cancer: The end of the beginning?. N. Engl. J. Med. 316, 939–941. https://doi.org/10.1056/NEJM198704093161509 (1987).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668. https://doi.org/10.1038/s41577-020-0306-5 (2020).
Gou, Q. et al. PD-L1 degradation pathway and immunotherapy for cancer. Cell Death Dis. 11, 955. https://doi.org/10.1038/s41419-020-03140-2 (2020).
Han, Y., Liu, D. & Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 10, 727–742 (2020).
Jiang, Y., Chen, M., Nie, H. & Yuan, Y. PD-1 and PD-L1 in cancer immunotherapy: Clinical implications and future considerations. Hum. Vaccin. Immunother. 15, 1111–1122. https://doi.org/10.1080/21645515.2019.1571892 (2019).
Zhao, Y. et al. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol. Biochem. 47, 721–734. https://doi.org/10.1159/000490025 (2018).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: Similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98–106. https://doi.org/10.1097/COC.0000000000000239 (2016).
Zhang, H. et al. Regulatory mechanisms of immune checkpoints PD-L1 and CTLA-4 in cancer. J. Exp. Clin. Cancer Res. 40, 184. https://doi.org/10.1186/s13046-021-01987-7 (2021).
Carroll, R. G. et al. An unexpected link between fatty acid synthase and cholesterol synthesis in proinflammatory macrophage activation. J. Biol. Chem. 293, 5509–5521. https://doi.org/10.1074/jbc.RA118.001921 (2018).
Sun, J. L. et al. Tumor cell-imposed iron restriction drives immunosuppressive polarization of tumor-associated macrophages. J. Transl. Med. 19, 347. https://doi.org/10.1186/s12967-021-03034-7 (2021).
Li, L. et al. A unique role for p53 in the regulation of M2 macrophage polarization. Cell Death Differ. 22, 1081–1093. https://doi.org/10.1038/cdd.2014.212 (2015).
Shi-Lin, D., Yuan, X., Zhan, S., Luo-Jia, T. & Chao-Yang, T. Trametinib, a novel MEK kinase inhibitor, suppresses lipopolysaccharide-induced tumor necrosis factor (TNF)-alpha production and endotoxin shock. Biochem. Biophys. Res. Commun. 458, 667–673. https://doi.org/10.1016/j.bbrc.2015.01.160 (2015).
Hollmen, M. et al. G-CSF regulates macrophage phenotype and associates with poor overall survival in human triple-negative breast cancer. Oncoimmunology 5, e1115177. https://doi.org/10.1080/2162402X.2015.1115177 (2016).
Acknowledgements
We are grateful to all of those with whom we have had the pleasure to work during this and other related projects.
Funding
The research was funded by the National Natural Science Foundation of China under Grant No. 82001223, the National Natural Science Foundation of China under grant no. 81901401, and the Natural Science Foundation for Young Scientist of Hunan Province, China (Grant No. 2019JJ50952, No. 2019JJ50948).
Author information
Authors and Affiliations
Contributions
J.Z. and Y.K. designed and performed the research and wrote the manuscript. Data curation and validation were performed by S.L. and F.L. J.Z. and Y.K. finished the revision. All authors contributed to writing and critically revising the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Li, S., Liu, F. et al. Role of CD68 in tumor immunity and prognosis prediction in pan-cancer. Sci Rep 12, 7844 (2022). https://doi.org/10.1038/s41598-022-11503-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-11503-2
This article is cited by
-
Antibiotics treatment promotes vasculogenesis in the brain of glioma-bearing mice
Cell Death & Disease (2024)
-
Genome-wide landscape of runs of homozygosity and differentiation across Egyptian goat breeds
BMC Genomics (2023)
-
Construction and validation of a novel prognostic model of neutrophil‑related genes signature of lung adenocarcinoma
Scientific Reports (2023)
-
Exploring scavenger receptor class F member 2 and the importance of scavenger receptor family in prediagnostic diseases
Toxicological Research (2023)
-
A Noninvasive Approach to Evaluate Tumor Immune Microenvironment and Predict Outcomes in Hepatocellular Carcinoma
Phenomics (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.