Article
|
Open Access
Featured
-
-
Article |
 Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres
Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres
Chiral 1,2-benzazaborines are promising isosteres of naphthalene, but rarely explored due to the lack of efficient synthetic methods. Now, the copper-catalysed enantioselective hydroboration of alkenes with 1,2-benzazaborines has been developed, providing a general platform for the atom-economic and efficient construction of diverse chiral 1,2-benzazaborine compounds bearing a 2-carbon-stereogenic centre or allene skeleton.
- Wanlan Su
- , Jide Zhu
- & Qiuling Song
-
Article
| Open Access A directed enolization strategy enables by-product-free construction of contiguous stereocentres en route to complex amino acids
A directed enolization strategy enables by-product-free construction of contiguous stereocentres en route to complex amino acids
α-Amino acids possessing β-stereocentres are difficult to synthesize. Now, an iridium-catalysed protocol allows the direct upconversion of simple alkenes and glycine derivatives to give β-substituted α-amino acids with exceptional levels of regio- and stereocontrol. The reaction design is based on exploiting the native directing ability of a glycine-derived N–H unit to facilitate enolization of the adjacent carbonyl.
- Fenglin Hong
- , Timothy P. Aldhous
- & John F. Bower
-
Article |
 Photoinduced dual bond rotation of a nitrogen-containing system realized by chalcogen substitution
Photoinduced dual bond rotation of a nitrogen-containing system realized by chalcogen substitution
Although photoinduced concerted multiple-bond-rotation processes are known in photoactive biological systems, the synthesis of compounds exhibiting similar behaviour has proven challenging. Now a thioamide-based system featuring chalcogen substituents has been shown to exhibit photoinduced C–N/C–C rotation; the rotation mode can be switched depending on external stimuli such as temperature and light irradiation.
- Shotaro Nagami
- , Rintaro Kaguchi
- & Akira Katsuyama
-
News & Views |
 Asymmetric construction of sulfur(VI)–fluorine cores
Asymmetric construction of sulfur(VI)–fluorine cores
Molecules containing a chiral S(VI) moiety have found extensive applications in drug design and organic synthesis, despite a lack of diverse asymmetric methods for their creation. Now, a ligand-mediated process has enabled the production of enantioenriched S(VI)–F motifs, providing a foundation for further stereospecific elaborations.
- Patrick R. Melvin
-
Article |
 Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Enantioselective sulfur(VI) fluoride exchange reaction of iminosulfur oxydifluorides
Chirality is an intrinsic property in unsymmetric three-dimensional molecular assembly, contributing to the utility of the corresponding process and the resulting scaffolds. Now, on the sulfur(VI) hub, a three-step sequential ligand-exchange method has been established with precise stereocontrol, enabling the enantioselective synthesis of optically active S(VI) functional molecules.
- Zhiyuan Peng
- , Shoujun Sun
- & Bing Gao
-
News & Views |
 Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Boron-enabled 1,3-metallate shift towards axially chiral alkenes
Tetracoordinate boron molecules are the key intermediates in many organoboron-related chemical transformations. Now, using alkynyl tetracoordinate boron species, a nickel-catalysed asymmetric 1,3-metallate shift towards axial chirality has been developed, giving access to various axially chiral alkenes in high efficiency.
- Li-Qing Ren
- & Chuan He
-
Article |
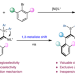 Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Ni-catalysed assembly of axially chiral alkenes from alkynyl tetracoordinate borons via 1,3-metallate shift
Enantioselective transformations based on tetracoordinate borons are elusive. Now, an enantioselective nickel-catalysed strategy for the construction of axially chiral alkenes has been reported, via a 1,3-metallate shift of alkynyl tetracoordinate boron species. The reaction uses readily accessible starting materials and a cheap transition-metal catalyst, and the chemoselectivity, regioselectivity and atroposelectivity could be well controlled.
- Xingxing Ma
- , Mengwei Tan
- & Qiuling Song
-
News & Views |
 An open-shell molecule that exhibits conformational dynamics
An open-shell molecule that exhibits conformational dynamics
Open-shell organic molecules with properties that can be modulated by external stimuli are of interest for spintronics applications. Now, an overcrowded alkene with open-shell tetraradical character has been synthesized in which the interaction between the π-conjugated subunits depends on the charge and spin state.
- Yoshito Tobe
-
Article |
 A catenane that is topologically achiral despite being composed of oriented rings
A catenane that is topologically achiral despite being composed of oriented rings
Catenanes are topologically non-trivial and, perhaps for this reason, molecules composed of two oriented rings have always simply been referred to as ‘topologically chiral’. Now it has been shown that the same stereogenic unit can arise in systems whose stereochemistry is Euclidean, suggesting a need to rethink this fundamental form of mechanical chirality.
- Noel Pairault
- , Federica Rizzi
- & Stephen M. Goldup
-
Article
| Open Access Control of dynamic sp3-C stereochemistry
Control of dynamic sp3-C stereochemistry
Stereogenic sp3-hybridized carbon centres are the principal building blocks of chiral organic molecules. Usually, these centres are configurationally fixed. Now, low-energy pericyclic rearrangements have been used to create rigid cage molecules with fluxional sp3-stereochemistry, influencing chiral information transfer. The sp3-carbon stereochemistry of the cages is inverted through strain-assisted Cope rearrangements.
- Aisha N. Bismillah
- , Toby G. Johnson
- & Paul R. McGonigal
-
News & Views |
 Chiral sulfinyls from sulfones
Chiral sulfinyls from sulfones
The selective removal of one oxygen atom from sulfones, without over-reduction to sulfide, is a challenging task. Now, through organocatalysis and incorporation of a cyano group into the sulfone, an asymmetric deoxygenation strategy has been developed, providing an efficient method for the synthesis of chiral sulfinyl compounds.
- Elżbieta Wojaczyńska
- & Jacek Wojaczyński
-
Article |
 Stereocontrolled acyclic diene metathesis polymerization
Stereocontrolled acyclic diene metathesis polymerization
Polymerization methods that control the cis/trans stereochemistry of repeating alkenes in polyalkenamers remain scarce. Now, an acyclic diene metathesis process has been developed that enables control over the stereochemistry of the polymer backbone. The method harnesses the reactivity of dithiolate Ru carbenes, in combination with cis,cis-diene monomers, to access several classes of polymers with tailored properties.
- Ting-Wei Hsu
- , Samuel J. Kempel
- & Quentin Michaudel
-
Article |
 Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Despite mechanically axially chiral (MAC) catenanes being recognized in 1961, their stereoselective synthesis had not been disclosed until now. Closer inspection of the MAC stereogenic unit has also led to the identification of an analogous, but unremarked upon, form of rotaxane stereochemistry and the conceptualization of a general approach to prepare MAC molecules stereoselectively.
- John R. J. Maynard
- , Peter Gallagher
- & Stephen M. Goldup
-
Article |
 Bottlebrush polymers with flexible enantiomeric side chains display differential biological properties
Bottlebrush polymers with flexible enantiomeric side chains display differential biological properties
The contributions of chirality and conformation as contributing factors to the biological properties of synthetic nanomaterials remain underexplored. A synthesis of bottlebrush polymers with mirror-image side chains has now been developed and it has been revealed that an interplay between side-chain absolute configuration and flexibility influences the biological properties of these polymers both in vitro and in vivo.
- Hung V.-T. Nguyen
- , Yivan Jiang
- & Jeremiah A. Johnson
-
Article |
 Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers
Olefin metathesis-based chemically recyclable polymers enabled by fused-ring monomers
Depolymerizable polymers can potentially address challenges in polymer sustainability, but most existing systems lack the useful thermomechanical properties of traditional ones. Now, it has been shown that depolymerizable polymers based on olefin metathesis show good thermal stability as well as versatile mechanical properties and that the monomers used to make them can be prepared from abundant materials.
- Devavrat Sathe
- , Junfeng Zhou
- & Junpeng Wang
-
Article |
 Catalytic asymmetric C–C cross-couplings enabled by photoexcitation
Catalytic asymmetric C–C cross-couplings enabled by photoexcitation
A chiral (η3-allyl)iridium(iii) complex has previously been used to catalyse enantioselective allylic substitution reactions in the polar domain. Now, it has been shown that the visible-light excitation of this iridium complex unlocks an otherwise inaccessible radical-based pathway to achieve enantioselective alkyl–alkyl cross-coupling reactions between allylic alcohols and radical precursors.
- Giacomo E. M. Crisenza
- , Adriana Faraone
- & Paolo Melchiorre
-
Article |
 Quaternary stereocentres via catalytic enantioconvergent nucleophilic substitution reactions of tertiary alkyl halides
Quaternary stereocentres via catalytic enantioconvergent nucleophilic substitution reactions of tertiary alkyl halides
A wide variety of bioactive molecules contain stereogenic quaternary carbons, and developing methods for the construction of these stereocentres continues to be an active area of research. Now, it has been shown that a nickel-catalysed enantioconvergent coupling of tertiary alkyl electrophiles with alkenylmetal nucleophiles—which probably proceeds via a radical pathway—can form and set quaternary stereocentres efficiently under mild conditions.
- Zhaobin Wang
- , Ze-Peng Yang
- & Gregory C. Fu
-
Review Article |
 Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates
Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates
The past decade has seen unprecedented growth in the development of chemical methods that proceed by mechanisms involving radical intermediates, but controlling absolute stereochemistry has been a longstanding challenge in this area. This Review Article examines how attractive non-covalent interactions between a chiral catalyst and the substrate can exert enantiocontrol in radical reactions.
- Rupert S. J. Proctor
- , Avene C. Colgan
- & Robert J. Phipps
-
Article |
 Catalytic enantiocontrol over a non-classical carbocation
Catalytic enantiocontrol over a non-classical carbocation
In 1949, Winstein and Trifan proposed that the 2-norbornyl cation adopts a bridged, non-classical structure. Now, the generation of an asymmetric environment around the three-centre two-electron bond of such an ion has been reported, enabling highly enantioselective catalytic addition reactions to a simple, non-functionalized non-classical cation.
- Roberta Properzi
- , Philip S. J. Kaib
- & Benjamin List
-
Article |
 Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Stereoselective access to [5.5.0] and [4.4.1] bicyclic compounds through Pd-catalysed divergent higher-order cycloadditions
Using readily accessible tropones and γ-methylidene-δ-valerolactones, the divergent synthesis of three classes of challenging [5.5.0] or [4.4.1] bicyclic systems has been achieved—with high efficiency and stereoselectivity—through Pd-catalysed higher-order cycloaddition. Mechanistic studies and density functional theory calculations indicate that the divergent reactions arise from the different reactivity of two diastereomeric intermediates.
- Li-Cheng Yang
- , Ya-Nong Wang
- & Yu Zhao
-
Article |
 Enantioselective radical C–H amination for the synthesis of β-amino alcohols
Enantioselective radical C–H amination for the synthesis of β-amino alcohols
The synthesis of chiral amino alcohols from simple alcohol starting materials can be achieved through a catalytic, asymmetric radical relay. Multiple catalysts work in concert to harness a H-atom transfer mechanism to enable enantio- and regioselective C–H amination by transient N-centred radicals.
- Kohki M. Nakafuku
- , Zuxiao Zhang
- & David A. Nagib
-
Article |
 Ruthenium-catalysed multicomponent synthesis of the 1,3-dienyl-6-oxy polyketide motif
Ruthenium-catalysed multicomponent synthesis of the 1,3-dienyl-6-oxy polyketide motif
A ruthenium-catalysed multicomponent reaction provides rapid and tunable access to 1,3-dienyl-6-oxy polyketide motifs. An initial alkene–alkyne coupling produces unsymmetrical 3-boryl-1,4-dienes. Allylation of aldehydes and ketones with these products is highly diastereoselective and results in the formation of two carbon–carbon bonds, two stereodefined olefins and up to three contiguous sp3 stereocentres.
- Barry M. Trost
- , James J. Cregg
- & Jacob S. Tracy
-
Article |
 Demystifying the asymmetry-amplifying, autocatalytic behaviour of the Soai reaction through structural, mechanistic and computational studies
Demystifying the asymmetry-amplifying, autocatalytic behaviour of the Soai reaction through structural, mechanistic and computational studies
The discovery of amplifying autocatalysis in a pyridine-3-carbaldehyde system facilitates a mechanistic deconstruction of the Soai reaction. A tetrameric autocatalyst, assembled by a combination of steric effects and nitrogen–zinc coordination, activates the substrate by two-point binding. This is followed by intra-complex isopropyl group transfer that generates the product alkoxide with high homochiral fidelity.
- Soumitra V. Athavale
- , Adam Simon
- & Scott E. Denmark
-
Article |
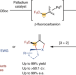 Use of α-trifluoromethyl carbanions for palladium-catalysed asymmetric cycloadditions
Use of α-trifluoromethyl carbanions for palladium-catalysed asymmetric cycloadditions
Although methods exist for the construction of CF3-containing stereocentres, the utilization of α-trifluoromethyl carbanions remains challenging because of the propensity for fluoride elimination. A strategy has now been developed to stabilize these carbanions through a neighbouring cationic Pd complex and the corresponding Pd-stabilized zwitterions participate in asymmetric cycloadditions with a broad range of acceptors.
- Barry M. Trost
- , Youliang Wang
- & Chao-I (Joey) Hung
-
Article |
 A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling
A general asymmetric copper-catalysed Sonogashira C(sp3)–C(sp) coupling
Asymmetric Sonogashira C(sp3)–C(sp) couplings provide complementary approaches to established C(sp3)–C(sp2/sp3) couplings for chiral C–C bond formation; however, relatively few reactions have been developed. Now, a versatile, enantioconvergent Sonogashira coupling via a radical intermediate has been developed. The approach uses a copper catalyst featuring a multidentate electron-rich cinchona alkaloid-derived ligand.
- Xiao-Yang Dong
- , Yu-Feng Zhang
- & Xin-Yuan Liu
-
Thesis |
 Double vision
Double vision
Michelle Francl wonders if a molecule can be just a little bit chiral?
- Michelle Francl
-
Article |
 Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Unravelling mechanistic features of organocatalysis with in situ modifications at the secondary sphere
Secondary-sphere interactions serve a fundamental role in controlling the reactivity and selectivity of organometallic and enzyme catalysts, but their study in organocatalytic systems is scarce. Now, it has been shown that the in situ secondary-sphere modification of organocatalysts combined with machine-learning techniques can uncover reaction mechanisms and streamline catalyst optimization.
- Vasudevan Dhayalan
- , Santosh C. Gadekar
- & Anat Milo
-
Article |
 E- and Z-, di- and tri-substituted alkenyl nitriles through catalytic cross-metathesis
E- and Z-, di- and tri-substituted alkenyl nitriles through catalytic cross-metathesis
Nitriles are widely used in chemistry and are found in many bioactive compounds but, despite recent advances, there are no broadly applicable approaches for the synthesis of di- or tri-substituted alkenyl nitriles. New catalytic cross-metathesis strategies now allow direct access to a wide range of Z- or E-di-substituted cyano-substituted alkenes and their tri-substituted variants.
- Yucheng Mu
- , Thach T. Nguyen
- & Amir H. Hoveyda
-
News & Views |
 Strained boronates do the trick
Strained boronates do the trick
Strained boronate complexes have now been shown to enable an unprecedented cross-coupling reaction across a C–C σ-bond. Using this approach, highly functionalized cyclobutanes can be prepared with excellent stereocontrol from readily available reagents.
- Alejandro Parra
- & Mariola Tortosa
-
Article |
 The formation of all-cis-(multi)fluorinated piperidines by a dearomatization–hydrogenation process
The formation of all-cis-(multi)fluorinated piperidines by a dearomatization–hydrogenation process
Despite their huge potential in medicinal chemistry, current approaches for the synthesis of fluorinated piperidines are often impractical. A straightforward process for the rhodium-catalysed dearomatization–hydrogenation of fluoropyridines has now been described. This strategy enables the highly diastereoselective formation of a variety of all-cis-(multi)fluorinated piperidines and the study of their conformational behaviour.
- Zackaria Nairoukh
- , Marco Wollenburg
- & Frank Glorius
-
Article |
 Carbopalladation of C–C σ-bonds enabled by strained boronate complexes
Carbopalladation of C–C σ-bonds enabled by strained boronate complexes
Widely used palladium-mediated cross-couplings typically operate via a handful of fundamental reaction steps. Now, the reactivity between palladium and C–C σ-bonds has been described. This carbopalladation enables the coupling of organoboronic esters and aryl triflates across a C–C σ-bond of a bicyclo[1.1.0]butane to form disastereomerically pure trisubstituted cyclobutanes.
- Alexander Fawcett
- , Tobias Biberger
- & Varinder K. Aggarwal
-
News & Views |
 What tangled webs we weave
What tangled webs we weave
Knots have been rigorously studied since the 1860s, but only in the past 30 years have they been made in the laboratory in molecular form. Now, the most complex small-molecule examples so far — a composite knot and an isomeric link, each with nine crossings — have been prepared.
- Edward E. Fenlon
-
News & Views |
 Easy as one, two, three
Easy as one, two, three
The preparation of three-dimensional frameworks with multiple stereocentres from simple acyclic hydrocarbons represents a challenging transformation. Now, starting from simple and readily available reagents, formation of these complex targets can be achieved in just three catalytic transformations with high levels of stereocontrol.
- Laura Castoldi
- & Vittorio Pace
-
Article |
 Efficient and stereodivergent synthesis of unsaturated acyclic fragments bearing contiguous stereogenic elements
Efficient and stereodivergent synthesis of unsaturated acyclic fragments bearing contiguous stereogenic elements
A rapid, modular, stereodivergent and diversity-oriented strategy for constructing acyclic molecular frameworks bearing up to four contiguous and congested stereogenic elements has been developed. This approach can yield the target compounds with remarkably high levels of stereocontrol in only three catalytic steps from commercially available alkynes.
- Jeffrey Bruffaerts
- , David Pierrot
- & Ilan Marek
-
Article |
 A new fundamental type of conformational isomerism
A new fundamental type of conformational isomerism
Isolable compounds displaying new fundamental forms of conformational isomerism were last discovered in 1914 (atropisomerism) and 1961 (hindered pyramidal inversion). Now, a new form—referred to as akamptisomerism—has been described through four resolved stereoisomers of a transoid (BF)O(BF)-quinoxalinoporphyrin compound. The stereodescriptors parvo and amplo, necessary for their classification, have also been introduced.
- Peter J. Canfield
- , Iain M. Blake
- & Maxwell J. Crossley
-
Article |
 Catalytic asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of sulfonium ylides
Catalytic asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of sulfonium ylides
The asymmetric Doyle–Kirmse reaction using chiral Rh(II)- or Cu(I)-catalysts provides SCF3-containing compounds in a highly efficient and enantioselective manner. The reaction proceeds through enantioselective formation of sulfonium ylide from a diazoester and allyl- or propargyl trifluoromethyl sulfide, followed by concerted [2,3]-sigmatropic rearrangement with the transfer of chirality from sulfur to carbon.
- Zhikun Zhang
- , Zhe Sheng
- & Jianbo Wang
-
Thesis |
 Pasteur and the art of chirality
Pasteur and the art of chirality
Louis Pasteur was a scientific giant of the nineteenth century, but, as Joseph Gal asks, was his most famous contribution to the understanding of chemistry — chirality — influenced more by his artistic talents?
- Joseph Gal
-
Article |
 Iterative assembly line synthesis of polypropionates with full stereocontrol
Iterative assembly line synthesis of polypropionates with full stereocontrol
Polypropionates can be grown — one carbon atom at a time — using the iterative homologation of boronic esters. This assembly line strategy was enabled through the use of enantioenriched lithiated α-chlorosilanes as masked carbinol units. Polypropionates were obtained in a fully stereocontrolled manner, including the stereochemically challenging anti–anti isomers.
- Teerawut Bootwicha
- , Julian M. Feilner
- & Varinder K. Aggarwal
-
News & Views |
 Single handedness in flatland
Single handedness in flatland
Planar molecules may break mirror symmetry when aligned on a surface, but both right- and left-handed forms will be created. Starting with a single-handed precursor, chiral adsorbates of planar hydrocarbons with a single handedness are formed in on-surface reactions.
- Karl-Heinz Ernst
-
Article |
 Switchable regioselectivity in amine-catalysed asymmetric cycloadditions
Switchable regioselectivity in amine-catalysed asymmetric cycloadditions
The construction of diversified compound libraries from identical substrates is attractive but remains a challenge in asymmetric synthesis. Here, we demonstrate switchable regioselective [6+2], [4+2] or [2+2] cycloadditions with α′-alkylidene-2-cyclopentenones via mild aminocatalysis, producing a spectrum of chiral frameworks with high structural diversity and molecular complexity.
- Zhi Zhou
- , Zhou-Xiang Wang
- & Ying-Chun Chen
-
Article |
 Organocatalytic stereoselective [8+2] and [6+4] cycloadditions
Organocatalytic stereoselective [8+2] and [6+4] cycloadditions
Higher-order cycloadditions — cycloadditions involving more than six π electrons — provide easy access to complex molecules containing heterocyclic or carbocyclic scaffolds. Now, it has been shown that aminocatalytic activation of 2-cycloalkenones affords mixtures of dienamines that can undergo [6 + 4] cycloadditions with heptafulvenes through a cross dienamine, or [8 + 2] cycloadditions through a linear dienamine.
- Rasmus Mose
- , Gert Preegel
- & Karl Anker Jørgensen
-
Article |
 By-design enantioselective self-amplification based on non-covalent product–catalyst interactions
By-design enantioselective self-amplification based on non-covalent product–catalyst interactions
Methods for preparing enantiomerically enriched products are often dependent on the structure of a catalyst. Here, it is shown that a self-amplifying catalyst is able to ‘sense’ the chirality of the catalytic product and induce enantioselectivity. Flexible ligand and product interaction sites are key to the increasing enantioselectivity over the course of the reaction.
- Golo Storch
- & Oliver Trapp
-
Article |
 Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides
Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides
Chiral, saturated N-heterocycles are prized as pharmaceutical agents and chiral auxiliaries, but are challenging to access without using prefunctionalized starting materials. Now, chiral phosphoric acids are found to enable the enantioselective Pd(II)-catalysed arylation of α-methylene C–H bonds in a wide variety of amines using thioamide as the directing group.
- Pankaj Jain
- , Pritha Verma
- & Jin-Quan Yu
-
Article |
 A chemically powered unidirectional rotary molecular motor based on a palladium redox cycle
A chemically powered unidirectional rotary molecular motor based on a palladium redox cycle
Control of motion at the molecular level is an integral requirement for the development of future nanoscale machinery. Now, governed by the fundamental reactivity principles of organometallic chemistry, a biaryl rotor is shown to exhibit 360° unidirectional rotary motion driven by the conversion of two simple fuels.
- Beatrice S. L. Collins
- , Jos C. M. Kistemaker
- & Ben L. Feringa
-
News & Views |
 A chiral catalyst with a ring to it
A chiral catalyst with a ring to it
A chiral [2]rotaxane in which the asymmetry is derived from the way in which the two components are mechanically interlocked — rather than being encoded in the covalent connectivity of the components themselves — has been shown to act as an enantioselective organocatalyst.
- Stephen M. Goldup
-
News & Views |
 Chiral cascades
Chiral cascades
Racemic or enantiomerically pure alcohols can be converted with high yield into enantiopure chiral amines in a one-pot redox-neutral cascade process by the clever combination of an alcohol dehydrogenase and an appropriate amine dehydrogenase.
- Jian-bo Wang
- & Manfred T. Reetz
-
Article |
 Diastereoselective addition of Grignard reagents to α-epoxy N-sulfonyl hydrazones
Diastereoselective addition of Grignard reagents to α-epoxy N-sulfonyl hydrazones
α-Substituted-β-hydroxy ketones are valuable intermediates, but their preparation by alkylation of enolates is difficult with hindered electrophiles. Now, a direct method for preparing α-substituted-β-hydroxy ketones — including those having α-quaternary centres — by addition of Grignard reagents to epoxyhydrazones has been developed, enabling the stereocontrolled incorporation of a wide range of carbon based substituents.
- Maulen M. Uteuliyev
- , Thien T. Nguyen
- & Don M. Coltart
-
Article |
 Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids
Rhodium-catalysed asymmetric allylic arylation of racemic halides with arylboronic acids
Cross-couplings between boronic acids and halides are a mainstay of synthetic organic chemistry but enantioselective
- Mireia Sidera
- & Stephen P. Fletcher
-
Article |
 Unidirectional rotary motion in achiral molecular motors
Unidirectional rotary motion in achiral molecular motors
Avoiding equal probability for clockwise and anticlockwise rotation is essential for the function of molecular motors, and both biological and synthetic systems take advantage of chirality to control the rotary direction. Now it has been shown, by integrating two rotor moieties in a symmetric meso motor design, that light-driven unidirectional rotary motion can be achieved in an achiral system.
- Jos C. M. Kistemaker
- , Peter Štacko
- & Ben L. Feringa

 Stereoretentive enantioconvergent reactions
Stereoretentive enantioconvergent reactions