Abstract
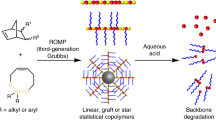
Chirality and molecular conformation are central components of life: biological systems rely on stereospecific interactions between discrete (macro)molecular conformers, and the impacts of stereochemistry and rigidity on the properties of small molecules and biomacromolecules have been intensively studied. Nevertheless, how these features affect the properties of synthetic macromolecules has received comparably little attention. Here we leverage iterative exponential growth and ring-opening metathesis polymerization to produce water-soluble, chiral bottlebrush polymers (CBPs) from two enantiomeric pairs of macromonomers of differing rigidity. Remarkably, CBPs with conformationally flexible, mirror image side chains show several-fold differences in cytotoxicity, cell uptake, blood pharmacokinetics and liver clearance; CBPs with comparably rigid, mirror image side chains show no differences. These observations are rationalized with a simple model that correlates greater conformational freedom with enhanced chiral recognition. Altogether, this work provides routes to the synthesis of chiral nanostructured polymers and suggests key roles for stereochemistry and conformational rigidity in the design of future biomaterials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information and can also be obtained from the corresponding author upon reasonable request.
References
Alberts, B. et al. Molecular Biology of the Cell 6th edn (Garland Science, 2014).
Bustamante, C., Cheng, W. & Mejia, Y. X. Revisiting the central dogma one molecule at a time. Cell 144, 480–497 (2011).
Schneider-Poetsch, T. & Yoshida, M. Along the central dogma—controlling gene expression with small molecules. Annu. Rev. Biochem. 87, 391–420 (2018).
Breslow, R. Artificial enzymes. Science 218, 532–537 (1982).
Breslow, R. & Cheng, Z.-L. On the origin of terrestrial homochirality for nucleosides and amino acids. Proc. Natl Acad. Sci. USA 106, 9144–9146 (2009).
FDA’s policy statement on the development of new stereoisomeric drugs. Chirality 4, 338–340 (1992).
Smith, S. W. Chiral toxicology: it’s the same thing…only different. Toxicol. Sci. 110, 4–30 (2009).
Brooks, W. H., Guida, W. C. & Daniel, K. G. The significance of chirality in drug design and development. Curr. Top. Med. Chem. 11, 760–770 (2011).
Shi, J., Votruba, A. R., Farokhzad, O. C. & Langer, R. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 10, 3223–3230 (2010).
Kakkar, A., Traverso, G., Farokhzad, O. C., Weissleider, R. & Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 1, 0063 (2017).
Elsabahy, M., Heo, G. S., Lim, S.-M., Sun, G. & Wooley, K. L. Polymeric nanostructures for imaging and therapy. Chem. Rev. 115, 10967–11011 (2015).
Washington, M. A. et al. The impact of monomer sequence and stereochemistry on the swelling and erosion of biodegradable poly(lactic-co-glycolic acid) matrices. Biomaterials 117, 66–76 (2017).
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
Otsukaa, H., Nagasaki, Y. & Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 64, 246–255 (2012).
Knop, K., Hoogenboom, R., Fischer, D. & Schubert, U. S. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 49, 6288–6308 (2010).
Kolate, A. et al. PEG—a versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 192, 67–81 (2014).
Kiick, K. L., Saxon, E., Tirrell, D. A. & Bertozzi, C. R. Incorporation of azides into recombinant proteins for chemoselective modification by the Staudinger ligation. Proc. Natl Acad. Sci. USA 99, 19–24 (2002).
Johnson, J. A., Lu, Y. Y., van Deventer, J. A. & Tirrell, D. A. Residue-specific incorporation of non-canonical amino acids into proteins: recent developments and applications. Curr. Opin. Chem. Biol. 14, 774–780 (2010).
van Hest, J. C. M. & Tirrell, D. A. Protein-based materials, toward a new level of structural control. Chem. Commun. 0, 1897–1904 (2001).
Lutz, J.-F., Ouchi, M., Liu, D. R. & Sawamoto, M. Sequence-controlled polymers. Science 341, 1238149 (2013).
Simon, R. J. et al. Peptoids: a modular approach to drug discovery. Proc. Natl Acad. Sci. USA 89, 9367–9371 (1992).
Wender, P. A. et al. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl Acad. Sci. USA 97, 13003–13008 (2000).
Fowler, S. A. & Blackwell, H. E. Structure–function relationships in peptoids: recent advances toward deciphering the structural requirements for biological function. Org. Biomol. Chem. 7, 1508–1524 (2009).
Gestwicki, J. E., Cairo, C. W., Strong, L. E., Oetjen, K. A. & Kiessling, L. L. Influencing receptor–ligand binding mechanisms with multivalent ligand architecture. J. Am. Chem. Soc. 124, 14922–14933 (2002).
Yeom, J. et al. Chiral supraparticles for controllable nanomedicine. Adv. Mater. 32, 1903878 (2020).
Wang, X., Gan, H. & Sun, T. Chiral design for polymeric biointerface: the influence of surface chirality on protein adsorption. Adv. Func. Mater. 21, 3276–3281 (2011).
Pooga, M. et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 16, 857–861 (1998).
Pattni, B. S., Chupin, V. V. & Torchilin, V. P. New developments in liposomal drug delivery. Chem. Rev. 115, 10938–10966 (2015).
Metselaar, J. M. et al. A novel family of l-amino acid-based biodegradable polymer−lipid conjugates for the development of long-circulating liposomes with effective drug-targeting capacity. Bioconjug. Chem. 14, 1156–1164 (2003).
Gaspar, M. M., Perez-Soler, R. & Cruz, M. E. Biological characterization of l-asparaginase liposomal formulations. Cancer Chemother. Pharmacol. 38, 373–377 (1996).
Sheiko, S. S., Sumerlin, B. S. & Matyjaszewski, K. Cylindrical molecular brushes: synthesis, characterization, and properties. Prog. Polym. Sci. 33, 759–785 (2008).
Verduzco, R., Li, X., Pesek, S. L. & Stein, G. E. Structure, function, self-assembly, and applications of bottlebrush copolymers. Chem. Soc. Rev. 44, 2405–2420 (2015).
Bielawski, C. W. & Grubbs, R. H. Living ring-opening metathesis polymerization. Prog. Polym. Sci. 32, 1–29 (2007).
Johnson, J. A. et al. Drug-loaded, bivalent-bottle-brush polymers by graft-through ROMP. Macromolecules 43, 10326–10335 (2010).
Nguyen, H. V.-T. et al. Nitroxide-based macromolecular contrast agents with unprecedented transverse relaxivity and stability for magnetic resonance imaging of tumors. ACS Cent. Sci. 3, 800–811 (2017).
Barnes, J. C. et al. Using an RNAi signature assay to guide the design of three-drug-conjugated nanoparticles with validated mechanisms, in vivo efficacy, and low toxicity. J. Am. Chem. Soc. 138, 12494–12501 (2016).
Binauld, S., Damiron, D., Connal, L. A., Hawker, C. J. & Drockenmuller, E. Precise synthesis of molecularly defined oligomers and polymers by orthogonal iterative divergent/convergent approaches. Macromol. Rapid Commun. 32, 147–168 (2011).
Barnes, J. C. et al. Iterative exponential growth of stereo- and sequence-controlled polymers. Nat. Chem. 7, 810–815 (2015).
Jiang, Y. et al. Iterative exponential growth synthesis and assembly of uniform diblock copolymers. J. Am. Chem. Soc. 138, 9369–9372 (2016).
Golder, M. R. et al. Stereochemical sequence dictates unimolecular diblock copolymer assembly. J. Am. Chem. Soc. 140, 1596–1599 (2018).
Pérez-Hernández, G., Paul, F., Giorgino, T., De Fabritiis, G. & Noé, F. Identification of slow molecular order parameters for Markov model construction. J. Chem. Phys. 139, 15102 (2013).
Schwantes, C. R. & Pande, V. S. Improvements in Markov state model construction reveal many non-native interactions in the folding of NTL9. J. Chem. Theory Comput. 9, 2000–2009 (2013).
Bowman, G. R. Improved coarse-graining of Markov state models via explicit consideration of statistical uncertainty. J. Chem. Phys. 137, 134111 (2012).
Dryzun, C., Zait, A. & Avnir, D. Quantitative symmetry and chirality—a fast computational algorithm for large structures: proteins, macromolecules, nanotubes, and unit cells. J. Comput. Chem. 32, 2526–2538 (2011).
Sowers, M. A. et al. Redox-responsive branched-bottlebrush polymers for in vivo MRI and fluorescence imaging. Nat. Commun. 5, 5460 (2014).
Johnson, J. A. et al. Core-clickable peg-branch-azide bivalent-bottle-brush polymers by romp: grafting-through and clicking-to. J. Am. Chem. Soc. 133, 559–566 (2011).
Choi, J., Reipa, V., Hitchins, V. M., Goering, P. L. & Malinauskas, R. A. Physicochemical characterization and in vitro hemolysis evaluation of silver nanoparticles. Toxicol. Sci. 123, 133–143 (2011).
Yu, B. et al. A novel star like eight-arm polyethylene glycol–deferoxamine conjugate for iron overload therapy. Pharmaceutics 12, 329 (2020).
Pachara, S. et al. Cytocompatibility evaluation of a novel series of PEG-functionalized lactide–caprolactone copolymer biomaterials for cardiovascular applications. Front. Bioeng. Biotechnol. 8, 991 (2020).
Rowland, M., Benet, L. Z. & Graham, G. G. Clearance concepts in pharmacokinetics. J. Pharmacokinet. Biopharm. 1, 123–136 (1973).
Acknowledgements
We thank the Army Research Office (W911NF-17-1-0521) and the National Institutes of Health (R01-CA220468-01 and postdoctoral fellowships for M.R.G. and N.J.O.) for supporting this work. We thank the National Science Foundation (graduate research fellowship for H.V.-T.N.). This work was supported in part by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute. We acknowledge the support of the National Institute of Standards and Technology, US Department of Commerce, in providing the neutron research facilities used in this work. We also thank J. Zhao for assistance with Fig. 1. We thank S.E. Denmark and A.F. Zahrt for helpful discussions.
Author information
Authors and Affiliations
Contributions
J.A.J., H.V.-T.N., J.C.B. and Y.J. conceived the idea. H.V.-T.N., Y.J., J.C.B., W.W., K.K.C., Z.H. and K.Y. performed the chemical syntheses and characterizations. H.V.-T.N., Y.J. J.C.B., N.J.O., Q.C., W.W. and M.R.G. performed biological studies. S.M., S.A. and R.G.-B. conducted simulations. M.J.A.H. conducted SANS studies. D.S., Y.S., A.P.W. and J.A.J. designed and conducted coarse-grained simulations. J.A.J., H.V.-T.N. and Y.J. wrote the manuscript. All authors discussed the results and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Arthi Jayaraman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–90, Tables 1–3, discussion, materials/general experimental methods/instrumentations and synthetic protocols.
Rights and permissions
About this article
Cite this article
Nguyen, H.VT., Jiang, Y., Mohapatra, S. et al. Bottlebrush polymers with flexible enantiomeric side chains display differential biological properties. Nat. Chem. 14, 85–93 (2022). https://doi.org/10.1038/s41557-021-00826-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00826-8
This article is cited by
-
Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut–brain axis
Nature Aging (2023)
-
Digital micelles of encoded polymeric amphiphiles for direct sequence reading and ex vivo label-free quantification
Nature Chemistry (2023)
-
Achiral organoiodine-functionalized helical polyisocyanides for multiple asymmetric dearomative oxidations
Nature Communications (2023)
-
Spectroscopy finds chiral phonons
Nature Photonics (2022)
-
Precise Pentamers with Diverse Monomer Sequences and Their Thermal Properties
Chinese Journal of Polymer Science (2022)