Abstract
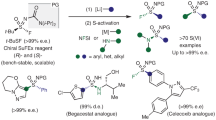
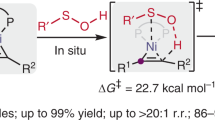
The trifluoromethylthio (SCF3) functional group has been of increasing importance in drug design and development as a consequence of its unique electronic properties and high stability coupled with its high lipophilicity. As a result, methods to introduce this highly electronegative functional group have attracted considerable attention in recent years. Although significant progress has been made in the introduction of SCF3 functionality into a variety of molecules, there remain significant challenges regarding the enantioselective synthesis of SCF3-containing compounds. Here, an asymmetric trifluoromethylthiolation that proceeds through the enantioselective [2,3]-sigmatropic rearrangement of a sulfonium ylide generated from a metal carbene and sulfide (Doyle–Kirmse reaction) has been developed using chiral Rh(II) and Cu(I) catalysts. This transformation features mild reaction conditions and excellent enantioselectivities (up to 98% yield and 98% e.e.), thus providing a unique, highly efficient and enantioselective method for the construction of C(sp3)–SCF3 bonds bearing chiral centres.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bégué, J.-P. & Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine (Wiley, 2008).
Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology (Blackwell, 2009).
Cametti, M., Crousse, B., Metrangolo, P., Milani, R. & Resnati, G. The fluorous effect in biomolecular applications. Chem. Soc. Rev. 41, 31–42 (2012).
Nakajima, T. Fluorine compounds as energy conversion materials. J. Fluorine Chem. 149, 104–111 (2013).
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506 (2014).
Chu, L. & Qing, F.-L. Oxidative trifluoromethylation and trifluoromethylthiolation reactions using (trifluoromethyl) trimethylsilane as a nucleophilic CF3 source. Acc. Chem. Res. 47, 1513–1522 (2014).
Toulgoat, F., Alazet, S. & Billard, T. Direct trifluoromethylthiolation reactions: the “renaissance” of an old concept. Eur. J. Org. Chem. 2415–2428 (2014).
Shao, X., Xu, C., Lu, L. & Shen, Q. Shelf-stable electrophilic reagents for trifluoromethylthiolation. Acc. Chem. Res. 48, 1227–1236 (2015).
Shen, C. et al. Recent advances in C–S bond formation via C–H bond functionalization and decarboxylation. Chem. Soc. Rev. 44, 291–314 (2015).
Xu, X.-H., Matsuzaki, K. & Shibata, N. Synthetic methods for compounds having CF3–S units on carbon by trifluoromethylation, trifluoromethylthiolation, triflylation, and related reactions. Chem. Rev. 115, 731–764 (2015).
Yang, X., Wu, T., Phipps, R. J. & Toste, F. D. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem. Rev. 115, 826–870 (2015).
Zhang, K., Xu, X. & Qing, F. Recent advances of direct trifluoromethylthiolation. Chin. J. Org. Chem. 35, 556–569 (2015).
Chachignon, H. & Cahard, D. State-of-the-art in electrophilic trifluoromethylthiolation reagents. Chin. J. Chem. 34, 445–454 (2016).
Bootwicha, T., Liu, X., Pluta, R., Atodiresei, I. & Rueping, M. N-Trifluoromethylthiophthalimide: a stable electrophilic SCF3-reagent and its application in the catalytic asymmetric trifluoromethylsulfenylation. Angew. Chem. Int. Ed. 52, 12856–12859 (2013).
Rueping, M., Liu, X., Bootwicha, T., Pluta, R. & Merkens, C. Catalytic enantioselective trifluoromethylthiolation of oxindoles using shelf-stable N-(trifluoromethylthio) phthalimide and a cinchona alkaloid catalyst. Chem. Commun. 50, 2508–2511 (2014).
Wang, X., Yang, T., Cheng, X. & Shen, Q. Enantioselective electrophilic trifluoromethylthiolation of β-ketoesters: a case of reactivity and selectivity bias for organocatalysis. Angew. Chem. Int. Ed. 52, 12860–12864 (2013).
Deng, Q.-H., Rettenmeier, C., Wadepohl, H. & Gade, L. H. Copper–boxmi complexes as highly enantioselective catalysts for electrophilic trifluoromethylthiolations. Chem. Eur. J. 20, 93–97 (2014).
Zhu, X.-L. et al. In situ generation of electrophilic trifluoromethylthio reagents for enantioselective trifluoromethylthiolation of oxindoles. Org. Lett. 16, 2192–2195 (2014).
Liu, X., An, R., Zhang, X., Luo, J. & Zhao, X. Enantioselective trifluoromethylthiolating lactonization catalyzed by an indane-based chiral sulfide. Angew. Chem. Int. Ed. 55, 5846–5850 (2016).
Kirmse, W. & Kapps, M. Reaktionen des diazomethans mit diallylsulfid und allyläthern unter kupfersalz-katalyse. Chem. Ber. 101, 994–1003 (1968).
Doyle, M. P., Griffin, J. H., Chinn, M. S. & van Leusen, D. Highly effective catalytic methods for ylide generation from diazo compounds. Mechanism of the rhodium-and copper-catalyzed reactions with allylic compounds. J. Org. Chem. 46, 5094–5102 (1981).
Li, A.-H., Dai, L.-X. & Aggarwal, V. K. Asymmetric ylide reactions: epoxidation, cyclopropanation, aziridination, olefination, and rearrangement. Chem. Rev. 97, 2341–2372 (1997).
Braverman, S. & Cherkinsky, M. [2,3]-Sigmatropic rearrangements of propargylic and allenic systems. Top. Curr. Chem. 275, 67 (2007).
Reggelin, M. [2,3]-Sigmatropic rearrangements of allylic sulfur compounds. Top. Curr. Chem. 275, 1–65 (2007).
Wang, J. in Comprehensive Organometallic Chemistry III Vol. 11 (eds Mingos, D. M. P. & Crabtree, R. H.) 151–178. (Applications II: Transition Metal Compounds In Organic Synthesis 2, Elsevier, 2007).
Zhang, Y. & Wang, J. Catalytic [2,3]-sigmatropic rearrangement of sulfur ylide derived from metal carbene. Coord. Chem. Rev. 254, 941–953 (2010).
West, T. H., Spoehrle, S. S. M., Kasten, K., Taylor, J. E. & Smith, A. D. Catalytic stereoselective [2,3]-rearrangement reactions. ACS Catal. 5, 7446–7479 (2015).
Nishibayashi, Y., Ohe, K. & Uemura, S. The first example of enantioselective carbenoid addition to organochalcogen atoms: application to [2,3]-sigmatropic rearrangement of allylic chalcogen ylides. Chem. Commun. 1245–1246 (1995).
Fukuda, T. & Katsuki, T. Co(III)-salen catalyzed carbenoid reaction: stereoselective [2,3]-sigmatropic rearrangement of S-ylides derived from allyl aryl sulfides. Tetrahedron Lett. 38, 3435–3438 (1997).
Itoh, K., Fukuda, T., Kitajima, H. & Katsuki, T. New aspect of carbenoid reaction: exploitation of new asymmetric synthesis using chiral carbenoid species. Yuki Gosei Kagaku Kyokaishi 55, 764–773 (1997).
Fukuda, T., Irie, R. & Katsuki, T. Catalytic and asymmetric [2,3]-sigmatropic rearrangement: Co(III)-salen catalyzed S-ylide formation from allyl aryl sulfides and their rearrangement. Tetrahedron 55, 649–664 (1999).
McMillen, D. W., Varga, N., Reed, B. A. & King, C. Asymmetric copper-catalyzed [2,3]-sigmatropic rearrangements of alkyl- and aryl-substituted allyl sulfides. J. Org. Chem. 65, 2532–2536 (2000).
Kitagaki, S., Yanamoto, Y., Okubo, H., Nakajima, M. & Hashimoto, S. Enantiocontrol in tandem allylic sulfonium ylide generation and [2,3]-sigmatropic rearrangement catalyzed by chiral dirhodium(II) complexes. Heterocycles 54, 623–628 (2001).
Zhang, X. et al. Catalytic asymmetric [2,3]-sigmatropic rearrangement of sulfur ylides generated from copper(I) carbenoids and allyl sulfides. J. Org. Chem. 67, 5621–5625 (2002).
Zhang, X., Ma, M. & Wang, J. Catalytic asymmetric [2,3]- sigmatropic rearrangement of sulfur ylides generated from carbenoids and propargyl sulfides. Tetrahedron: Asymm. 14, 891–895 (2003).
Zhang, X., Ma, M. & Wang, J. Catalytic asymmetric [2,3]-sigmatropic rearrangement of sulfur ylides generated from carbenoids and allenic 2-methylphenyl sulfide. Chin. J. Chem. 21, 878–882 (2003).
Ma, M., Peng, L., Li, C., Zhang, X. & Wang, J. Highly stereoselective [2,3]-sigmatropic rearrangement of sulfur ylide generated through Cu(I) carbene and sulfides. J. Am. Chem. Soc. 127, 15016–15017 (2005).
Liao, M. & Wang, J. Highly efficient [2,3]-sigmatropic rearrangement of sulfur ylide derived from Rh(II) carbene and sulfides in water. Green Chem. 9, 184–188 (2007).
Zhang, H., Wang, B., Yi, H., Zhang, Y. & Wang, J. Rh (II)-catalyzed [2, 3]-sigmatropic rearrangement of sulfur ylides derived from cyclopropenes and sulfides. Org. Lett. 17, 3322–3325 (2015).
Tyagi, V., Sreenilayam, G., Bajaj, P., Tinoco, A. & Fasan, R. Biocatalytic synthesis of allylic and allenyl sulfides through a myoglobin-catalyzed Doyle–Kirmse reaction. Angew. Chem. Int. Ed. 55, 13562–13566 (2016).
Doyle, M. P. & Forbes, D. C. Recent advances in asymmetric catalytic metal carbene transformations. Chem. Rev. 98, 911–935 (1998).
Davies, H. M. L. & Beckwith, R. E. J. Catalytic enantioselective C-H activation by means of metal-carbenoid-induced C-H insertion. Chem. Rev. 103, 2861–2903 (2003).
Davies, H. M. L. & Nikolai, J. Catalytic and enantioselective allylic C–H activation with donor–acceptor-substituted carbenoids. Org. Biomol. Chem. 3, 4176–4187 (2005).
Aggarwal, V. K., Ferrara, M. & Hainz, R. [2,3]-Sigmatropic rearrangement of allylic sulfur ylides derived from trimethylsilyldiazomethane (TMSD). Tetrahedron Lett. 40, 8923–8927 (1999).
Li, Z., Parr, B. T. & Davies, H. M. L. Highly stereoselective C−C bond formation by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and chiral allylic alcohols. J. Am. Chem. Soc. 134, 10942–10946 (2012).
Li, Z. et al. Scope and mechanistic analysis of the enantioselective synthesis of allenes by rhodium-catalyzed tandem ylide formation/[2,3]-sigmatropic rearrangement between donor/acceptor carbenoids and propargylic alcohols. J. Am. Chem. Soc. 134, 15497–15504 (2012).
Parr, B. T. & Davies, H. M. L. Highly stereoselective synthesis of cyclopentanes bearing four stereocentres by a rhodium carbene-initiated domino sequence. Nat. Commun. 5, 4455 (2014).
Trost, B. M. & Hammen, R. F. New synthetic methods. Transfer of chirality from sulfur to carbon. J. Am. Chem. Soc. 95, 962–964 (1973).
Trost, B. M. & Biddlecom, W. G. Asymmetric induction in a [2,3]-sigmatropic rearrangement. J. Org. Chem. 38, 3438–3439 (1973).
Davies, H. M. L. & Morton, D. Guiding principles for site selective and stereoselective intermolecular C–H functionalization by donor/acceptor rhodium carbenes. Chem. Soc. Rev. 40, 1857–1869 (2011).
Acknowledgements
The authors acknowledge financial support from the National Basic Research Program of China (973 programme no. 2015CB856600) and Natural Science Foundation of China (grant no. 21332002). We thank Y. Wang and Z. Yu (Peking University) for the discussion on the reaction mechanism. We greatly appreciate W.-X. Zhang and N. Wang (Peking University) for the assistance in obtaining X-ray crystallographic structures.
Author information
Authors and Affiliations
Contributions
Z.Z., Z.S., W.Y., G.W. and R.Z. performed the experiments. W.-D.C. and Y.Z. participated in the discussion and helped the measurement of optical purities. J.W. conceived and supervised the project. Z.Z. and J.W. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 9487 kb)
Supplementary information
Crystallographic data for compound 8 (CIF 37 kb)
Supplementary information
Crystallographic data for compound 10j (CIF 36 kb)
Rights and permissions
About this article
Cite this article
Zhang, Z., Sheng, Z., Yu, W. et al. Catalytic asymmetric trifluoromethylthiolation via enantioselective [2,3]-sigmatropic rearrangement of sulfonium ylides. Nature Chem 9, 970–976 (2017). https://doi.org/10.1038/nchem.2789
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.2789
This article is cited by
-
Electrochemical oxidative difunctionalization of diazo compounds with two different nucleophiles
Nature Communications (2023)
-
Design, synthesis, and applications of stereospecific 1,3-diene carbonyls
Science China Chemistry (2022)
-
Modular and stereoselective synthesis of tetrasubstituted vinyl sulfides leading to a library of AIEgens
Nature Communications (2021)
-
Transient-axial-chirality controlled asymmetric rhodium-carbene C(sp2)-H functionalization for the synthesis of chiral fluorenes
Nature Communications (2020)
-
Use of trifluoroacetaldehyde N-tfsylhydrazone as a trifluorodiazoethane surrogate and its synthetic applications
Nature Communications (2019)