Abstract
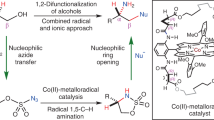
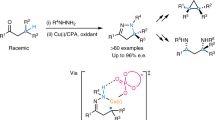
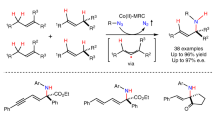
Asymmetric, radical C–H functionalizations are rare but powerful tools for solving modern synthetic challenges. Specifically, the enantio- and regioselective C–H amination of alcohols to access medicinally valuable chiral β-amino alcohols remains elusive. To solve this challenge, a radical relay chaperone strategy was designed, wherein an alcohol was transiently converted to an imidate radical that underwent intramolecular H-atom transfer (HAT). This regioselective HAT was also rendered enantioselective by harnessing energy transfer catalysis to mediate selective radical generation and interception by a chiral copper catalyst. The successful development of this multi-catalytic, asymmetric, radical C–H amination enabled broad access to chiral β-amino alcohols from a variety of alcohols containing alkyl, allyl, benzyl and propargyl C–H bonds. Mechanistic experiments revealed that triplet energy sensitization of a Cu-bound radical precursor facilitates catalyst-mediated HAT stereoselectivity, enabling the synthesis of several important classes of chiral β-amines by enantioselective, radical C–H amination.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information files.
References
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Park, Y., Kim, Y. & Chang, S. Transition metal-catalyzed C–H amination: scope, mechanism, and applications. Chem. Rev. 117, 9247–9301 (2017).
Davies, H. M. L. & Manning, J. R. Catalytic C–H functionalization by metal carbenoid and nitrenoid insertion. Nature 451, 417–424 (2008).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C–H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Paudyal, M. P. et al. Dirhodium-catalyzed C–H arene amination using hydroxylamines. Science 353, 1144–1147 (2016).
Ruffoni, A. et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 11, 426–433 (2019).
McNally, A., Haffemayer, B., Collins, B. S. L. & Gaunt, M. J. Palladium-catalysed C–H activation of aliphatic amines to give strained nitrogen heterocycles. Nature 510, 129–133 (2014).
Sharma, A. & Hartwig, J. F. Metal-catalysed azidation of tertiary C–H bonds suitable for late-stage functionalization. Nature 517, 600–604 (2015).
Hong, S. Y. et al. Selective formation of γ-lactams via C–H amidation enabled by tailored iridium catalysts. Science 359, 1016–1021 (2018).
Reddy, R. P. & Davies, H. M. L. Dirhodium tetracarboxylates derived from adamantylglycine as chiral catalysts for enantioselective C–H aminations. Org. Lett. 8, 5013–5016 (2006).
Zalatan, D. N. & Du Bois, J. A chiral rhodium carboxamidate catalyst for enantioselective C–H amination. J. Am. Chem. Soc. 130, 9220–9221 (2008).
Milczek, E., Boudet, N. & Blakey, S. Enantioselective C–H amination using cationic ruthenium(ii)–pybox catalysts. Angew. Chem. Int. Ed. 47, 6825–6828 (2008).
Park, Y. & Chang, S. Asymmetric formation of γ-lactams via C–H amidation enabled by chiral hydrogen-bond-donor catalysts. Nat. Catal. 2, 219–227 (2019).
Hu, Y. et al. Enantioselective radical construction of 5-membered cyclic sulfonamides by metalloradical C–H amination. J. Am. Chem. Soc. 141, 18160–18169 (2019).
Yang, Y., Cho, I., Qi, X., Liu, P. & Arnold, F. H. An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp 3)–H bonds. Nat. Chem. 11, 987–993 (2019).
Wappes, E. A., Nakafuku, K. M. & Nagib, D. A. Directed β C–H amination of alcohols via radical relay chaperones. J. Am. Chem. Soc. 139, 10204–10207 (2017).
Stateman, L. M., Nakafuku, K. M. & Nagib, D. A. Remote C–H functionalization via selective hydrogen atom transfer. Synthesis 50, 1569–1586 (2018).
Choi, G. J., Zhu, Q., Miller, D. C., Gu, C. J. & Knowles, R. R. Catalytic alkylation of remote C–H bonds enabled by proton-coupled electron transfer. Nature 539, 268–271 (2016).
Chu, J. C. K. & Rovis, T. Amide-directed photoredox-catalysed C–C bond formation at unactivated sp 3 C–H bonds. Nature 539, 272–275 (2016).
Twilton, J. et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 1, 0052 (2017).
Wang, F., Chen, P. & Liu, G. Copper-catalyzed radical relay for asymmetric radical transformations. Acc. Chem. Res. 51, 2036–2046 (2018).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Ager, D. J., Prakash, I. & Schaad, D. R. 1,2-Amino alcohols and their heterocyclic derivatives as chiral auxiliaries in asymmetric synthesis. Chem. Rev. 96, 835–875 (1996).
Robak, M. T., Herbage, M. A. & Ellman, J. A. Synthesis and applications of tert-butanesulfinamide. Chem. Rev. 110, 3600–3740 (2010).
Chang, H. T. & Sharpless, K. B. A practical route to enantiopure 1,2-aminoalcohols. Tetrahedron Lett. 37, 3219–3222 (1996).
Zhang, W. et al. Enantioselective cyanation of benzylic C–H bonds via copper-catalyzed radical relay. Science 353, 1014–1018 (2016).
Murphy, J. J., Bastida, D., Paria, S., Fagnoni, M. & Melchiorre, P. Asymmetric catalytic formation of quaternary carbons by iminium ion trapping of radicals. Nature 532, 218–222 (2016).
Wang, C., Harms, K. & Meggers, E. Catalytic asymmetric Csp 3−H functionalization under photoredox conditions by radical translocation and stereocontrolled alkene addition. Angew. Chem. Int. Ed. 55, 13495–13498 (2016).
Proctor, R. S. J., Davis, H. J. & Phipps, R. J. Catalytic enantioselective Minisci-type addition to heteroarenes. Science 360, 419–422 (2018).
Zhang, W., Wu, L., Chen, P. & Liu, G. Enantioselective arylation of benzylic C−H bonds by copper-catalyzed radical relay. Angew. Chem. Int. Ed. 58, 6425–6429 (2019).
Shin, N. Y., Ryss, J. M., Zhang, X., Miller, S. J. & Knowles, R. R. Light-driven deracemization enabled by excited-state electron transfer. Science 366, 364–369 (2019).
Li, J. et al. Site-specific allylic C–H bond functionalization with a copper-bound N-centred radical. Nature 574, 516–521 (2019).
Ni, Z. et al. Highly regioselective copper-catalyzed benzylic C–H amination by N-fluorobenzenesulfonimide. Angew. Chem. Int. Ed. 51, 1244–1247 (2012).
Luo, Y. R. Comprehensive Handbook of Chemical Bond Energies (CRC Press, 2007).
Griller, D., Ingold, K. U., Krusic, P. J. & Fischer, H. Configuration of the tert-butyl radical. J. Am. Chem. Soc. 100, 6750–6752 (1978).
Nakafuku, K. M., Fosu, S. C. & Nagib, D. A. Catalytic alkene difunctionalization via imidate radicals. J. Am. Chem. Soc. 140, 11202–11205 (2018).
Welin, E. R., Le, C., Arias-Rotondo, D. M., McCusker, J. K. & MacMillan, D. W. C. Photosensitized, energy transfer-mediated organometallic catalysis through electronically excited nickel(ii). Science 355, 380–385 (2017).
Gutierrez, O., Tellis, J. C., Primer, D. N., Molander, G. A. & Kozlowski, M. C. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 137, 4896–4899 (2015).
Kochi, J. K. Electron-transfer mechanisms for organometallic intermediates in catalytic reactions. Acc. Chem. Res. 7, 351–360 (1974).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Farney, E. P. et al. Discovery and elucidation of counteranion dependence in photoredox catalysis. J. Am. Chem. Soc. 141, 6385–6391 (2019).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Blum, T. R., Miller, Z. D., Bates, D. M., Guzei, I. A. & Yoon, T. P. Enantioselective photochemistry through Lewis acid-catalyzed triplet energy transfer. Science 354, 1391–1395 (2016).
Wang, Y.-F., Chen, H., Zhu, X. & Chiba, S. Copper-catalyzed aerobic aliphatic C–H oxygenation directed by an amidine moiety. J. Am. Chem. Soc. 134, 11980–11983 (2012).
Alderson, J. M., Corbin, J. R. & Schomaker, J. M. Tunable, chemo- and site-selective nitrene transfer reactions through the rational design of silver(i) catalysts. Acc. Chem. Res. 50, 2147–2158 (2017).
Korth, H. G., Trill, H. & Sustmann, R. [1-2H]-Allyl radical: barrier to rotation and allyl delocalization energy. J. Am. Chem. Soc. 103, 4483–4489 (1981).
Newcomb, M., Johnson, C. C., Manek, M. B. & Varick, T. R. Picosecond radical kinetics. Ring openings of phenyl-substituted cyclopropylcarbinyl radicals. J. Am. Chem. Soc. 114, 10915–10921 (1992).
Gloor, C. S., Dénès, F. & Renaud, P. Memory of chirality in reactions involving monoradicals. Free Radic. Res. 50, S102–S111 (2016).
Wüstenberg, B. & Pfaltz, A. Homogeneous hydrogenation of tri- and tetrasubstituted olefins: comparison of iridium–phospinooxazoline [Ir–PHOX] complexes and Crabtree catalysts with hexafluorophosphate (PF6) and tetrakis[3,5-bis(trifluoromethyl) phenyl]borate (BArF) as counterions. Adv. Synth. Catal. 350, 174–178 (2008).
Acknowledgements
Financial support was provided by the National Institutes of Health (NIH R35 GM119812) and National Science Foundation (NSF CAREER 1654656). L.M.S. is supported by an NSF graduate fellowship. Calculations were performed using resources at the Ohio Supercomputer Center.
Author information
Authors and Affiliations
Contributions
K.M.N. designed and discovered the enantioselective C–H amination. K.M.N., E.A.W. and Z.Z. developed the optimized method. Z.Z. evaluated the synthetic scope. Z.Z. and L.M.S. performed the mechanistic experiments and derivatizations. A.D.C. performed the calculations. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Tables 1–7, new compound characterization, and mechanistic and density functional theory experiments.
Rights and permissions
About this article
Cite this article
Nakafuku, K.M., Zhang, Z., Wappes, E.A. et al. Enantioselective radical C–H amination for the synthesis of β-amino alcohols. Nat. Chem. 12, 697–704 (2020). https://doi.org/10.1038/s41557-020-0482-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0482-8
This article is cited by
-
Palladium-catalysed methylene C(sp3)–H lactamization and cycloamination enabled by chlorinated pyridine–pyridone ligands
Nature Synthesis (2024)
-
Site-selective arene C–H amination with iron-aminyl radical
Nature Catalysis (2024)
-
Electrochemical carbon–carbon coupling with enhanced activity and racemate stereoselectivity by microenvironment regulation
Nature Communications (2023)
-
A general copper-catalysed enantioconvergent radical Michaelis–Becker-type C(sp3)–P cross-coupling
Nature Synthesis (2023)
-
Cu/photoredox-catalyzed decarboxylative radical C(sp3)-C(sp3) cross-coupling reactions
Science China Chemistry (2023)