« Prev Next »
DNA Fingerprinting Defined
In order to understand how AFLP-PCR works, it's first important to understand what researchers mean when they use the term "DNA fingerprinting." In short, DNA fingerprinting involves the generation of a set of distinct DNA fragments from a single DNA sample; these fragments are then used as a source of genotypic information. A wide range of techniques can be used to generate DNA fingerprinting data. Researchers choose among these techniques based on the organism being studied and on the question being addressed.
All DNA fingerprinting methods study patterns associated with genetic markers; however, individual techniques differ in terms of the number and type of genetic markers examined. For example, some approaches allow the examination of a marker at a single locus (called single-locus markers), whereas others allow the simultaneous investigation of multiple loci (called multilocus markers). Some approaches focus on codominant markers, which provide information about both alleles present at a given locus. In contrast, other techniques are concerned with dominant markers, which only report the presence or absence of a given allele and cannot provide information about whether an individual is homozygous for that allele.
The oldest method used in DNA fingerprinting studies is restriction fragment length polymorphism (RFLP) analysis. RFLP analysis has been widely employed by researchers to identify genes linked to several Mendelian (single-gene) diseases, such as Huntington's disease. This approach detects differences in DNA fragment lengths due to the presence or absence of a restriction enzyme site, or due to an insertion or deletion that occurs between two restriction enzyme sites. Although RFLP analysis does not require knowledge of the genome sequence, RFLP studies can be extremely time-consuming and challenging in the absence of such data. When sequence data is not available, the experimenter must physically clone a region of the genome under study that is large enough to be cut up by enzymes, and this process requires a great deal of time and resources.
As opposed to RFLP analysis, a second DNA fingerprinting technique focuses on microsatellite regions of the genome that contain simple sequence repeats (SSRs), which are short stretches of two to six nucleotides that are repeated multiple times. Most often, SSRs consist of two or three nucleotides (e.g., CACACACA...). The number of SSRs within a given microsatellite region of the genome often varies among individuals. Because of these differences, researchers can use PCR-based approaches with primers that flank the microsatellite region to determine the length of the SSR. The size of the resulting PCR products is determined using high-resolution gel electrophoresis. This approach requires knowledge of the genome sequence to design the primers, but it can be used to detect multiple alleles for a given microsatellite region.
Yet another approach to DNA fingerprinting, called random amplified polymorphic DNA (RAPD) analysis, uses PCR primer sets designed to randomly amplify DNA fragments scattered throughout the genome. RAPD is a multilocus approach that uses low-stringency PCR conditions to permit promiscuous amplification of genomic regions. It can be carried out in the absence of genome sequence data, but the low-stringency conditions sometimes provide challenges when trying to reproduce or interpret results.
Today, researchers are increasingly turning to AFLP-PCR in their DNA fingerprinting efforts. AFLP-PCR uses many of the same steps as RFLP, SSR, and RAPD, including restriction enzyme digestion of genomic DNA, PCR, and electrophoretic separation of DNA fragments. However, this method includes additional steps that permit high-resolution interrogation of the entire genome, and it yields highly specific, reproducible genotypic data. Although AFLP can only be used to study dominant genetic markers, it does not rely on any previous knowledge of the genome sequence.
A Closer Look at AFLP-PCR
AFLP-PCR was first described by researcher Pieter Vos and his colleagues in 1995 (Vos et al., 1995). This technique involves five major steps, as described in the following sections.
Step 1: Preparing the AFLP Template
Step 2: Ligation Reaction with Restriction Fragments and Adaptors
The aforementioned ligation reaction involves incubation of the MseI/EcoRI digested genomic DNA together with the two adaptors and with DNA ligase, which is an enzyme that covalently links the adaptors with their respective complementary 5′ ends. MseI and EcoRI restriction enzymes can also be added to this ligation reaction, as they will prevent re-ligation between EcoRI-EcoRI and MseI-MseI genomic fragments and will not recognize the ligated adaptor-genomic DNA fragments.
Step 3: Selective PCR Amplification
Although the MseI and EcoRI adaptor-ligated ends of each DNA fragment will be identical, the sequences of the genomic DNA fragments, which begin just after the original MseI and EcoRI sites, will differ. If researchers carried out PCR reactions using primers corresponding to the MseI and EcoRI adaptor sequences, they would amplify every single genomic DNA fragment, and they would therefore be faced with an indecipherable set of DNA fragments. Thus, in order to selectively amplify a smaller number of genomic DNA fragments, researchers use primer sets that are complementary to the MseI or EcoRI adaptor sequences starting at their 5′ ends but that add up to three unique nucleotides following the end of the original MseI or EcoRI recognition site. The addition of only a small number of base pairs, called selective nucleotides, at the end of each primer permits the amplification of a subset of genomic DNA fragments. The more complex the genome being investigated, the more selective nucleotides researchers add to the primers. Some simpler genomes might not require any selective nucleotides at all (Figure 2).
As the number of nucleotides added to the 3′ end of each primer is increased, the selectivity of the primer also increases. After all, more genomic DNA fragments will be amplified by a primer that contains a single unique base at its 3′ end compared to a primer that contains three unique bases at its 3′ end. Primer design does not require any previous knowledge of the genome under study. Rather, researchers simply set up PCR reactions using a variety of primer sets, each of which consists of one MseI-associated primer and one EcoRI-associated primer. AFLP-PCR reactions are carried out under stringent conditions, permitting only the selective amplification of those genomic DNA fragments that are perfectly complementary to the 3′ ends of the primer sequences. The stringent PCR conditions employed in AFLP-PCR reactions lead to highly reproducible results that are readily comparable among different samples.
Step 4: Electrophoretic Separation of Amplified DNA Fragments
Step 5: Analysis
Getting to Work: How to Use AFLP Data
Currently, AFLP data can be used to answer a wide range of genetic questions (Mueller & Wolfenbarger, 1999). This technique can be especially useful in addressing questions related to organisms whose genome sequence has not yet been determined, including many plants, fungi, and bacteria. AFLP can help indicate whether two organisms are members of the same species. Furthermore, it can be used to assess genetic variation within a species or among closely related species. Population geneticists also use AFLP approaches to determine genetic variation across different populations. Indeed, this method has allowed researchers to refine the taxonomic classification of organisms based on AFLP-associated genetic markers.
Of course, AFLP can also be used to study organisms whose genome has been sequenced. For example, AFLP has been used to study human DNA samples in criminal investigations and in paternity tests (Brinkman, 1991).
In the hospital setting, AFLP has been used to track pathogenic outbreaks to determine whether an outbreak is associated with the spread of a single strain or multiple distinct strains (Jonas et al., 2000). In this type of analysis, the pathogenic organism is isolated from infected patients and subjected to AFLP analysis. By comparing AFLP data collected from different patients, researchers can readily determine whether the outbreak stems from one or from many different strains.
New Directions
In addition to their widespread use in DNA fingerprinting, AFLP-based approaches have also been used to produce gene expression fingerprints. AFLP gene expression fingerprints are generated using cDNA (rather than genomic DNA) as the PCR template. With this approach, researchers can study gene expression from multiple loci as a means of comparison between two different individuals or populations. In the years to come, the uses of AFLP will continue to evolve as researchers are faced with an ever-expanding list of complex genetic questions.
References and Recommended Reading
Jonas, D., et al. Comparative evaluation of three different genotyping methods for investigation of nosocomial outbreaks of Legionnaires' disease in hospitals. Journal of Clinical Microbiology 38, 2284–2291 (2000)
Mueller, U. G., & Wolfenbarger, L. L. AFLP genotyping and fingerprinting. Trends in Ecology and Evolution 14, 389–394 (1999)
Vos, P., et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Research 23, 4407–4414 (1995)
Vuylsteke, M., et al. AFLP technology for DNA fingerprinting. Nature Protocols 2, 1387–1398 (2007) doi:10.1038/nprot.2007.175 (link to article)




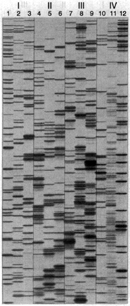
 Figure 1
Figure 1