Featured
-
-
News & Views |
 Stable organolithium gels
Stable organolithium gels
Organolithium reagents are characterized by their high reactivity towards air and moisture, traditionally requiring strict inert conditions for their handling and utilization. Now, these reagents can be encapsulated within an organogel, enhancing their stability and allowing their use and storage under ambient conditions.
- Andreu Tortajada
- & Eva Hevia
-
Article
| Open Access Organogel delivery vehicles for the stabilization of organolithium reagents
Organogel delivery vehicles for the stabilization of organolithium reagents
Organolithium compounds are important reagents but are often hazardous due to their high reactivity. Now, encapsulating organolithium reagents within a supramolecular organogel has been found to enhance their stability, facilitating their storage and handling under ambient conditions. The homogeneous gels can be easily subdivided and dosed into a wide range of synthetic reactions.
- Petr Slavík
- , Benjamin R. Trowse
- & David K. Smith
-
Article |
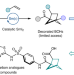 A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres
A catalytic alkene insertion approach to bicyclo[2.1.1]hexane bioisosteres
Substituted bicyclo[2.1.1]hexanes (BCHs) are emerging bicyclic hydrocarbon bioisosteres for ortho- and meta-substituted benzenes, but are difficult to access. Now a SmI2-catalysed intermolecular coupling of bicyclo[1.1.0]butyl ketones and alkenes provides a general approach to access substituted BCHs, thus promoting their widespread use in medicinal chemistry and crop science.
- Soumitra Agasti
- , Frédéric Beltran
- & David J. Procter
-
News & Views |
 Chiral sulfinyls from sulfones
Chiral sulfinyls from sulfones
The selective removal of one oxygen atom from sulfones, without over-reduction to sulfide, is a challenging task. Now, through organocatalysis and incorporation of a cyano group into the sulfone, an asymmetric deoxygenation strategy has been developed, providing an efficient method for the synthesis of chiral sulfinyl compounds.
- Elżbieta Wojaczyńska
- & Jacek Wojaczyński
-
News & Views |
 Interlocked structures on active duty
Interlocked structures on active duty
Interlocking macrocyclic carbon nanomaterials is an exciting way to tune their molecular properties, but all-conjugated catenanes and rotaxanes are extremely challenging to make. Now, fully π-conjugated [2]- and [3]catenanes as well as a [3]rotaxane have been prepared through an ‘active metal template’ approach.
- Satyajit Das
- & Fredrik Schaufelberger
-
Article
| Open Access Photoswitching neutral homoaromatic hydrocarbons
Photoswitching neutral homoaromatic hydrocarbons
Neutral homoaromatic hydrocarbons—which possess an interrupted π-system yet display aromatic properties owing to through-space or through-bond interactions—have remained rare as they are typically unstable. Now a class of stable neutral homoaromatic homoannulenes has been synthesized, including one that acts as a photoswitch through a reversible [1, 11] sigmatropic rearrangement.
- Trung Tran Ngoc
- , Niklas Grabicki
- & Johannes F. Teichert
-
Article |
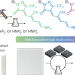 Mechanically gated formation of donor–acceptor Stenhouse adducts enabling mechanochemical multicolour soft lithography
Mechanically gated formation of donor–acceptor Stenhouse adducts enabling mechanochemical multicolour soft lithography
Mechanochemical generation of dyes with different photophysical properties generally requires the use of discrete mechanophore derivatives with unique chemical structures. Now it has been shown that diverse donor–acceptor Stenhouse adducts can be produced via a mechanically gated chromogenic reaction, enabling mechanochemical multicolour lithography.
- Anna C. Overholts
- , Wendy Granados Razo
- & Maxwell J. Robb
-
Article |
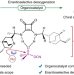 Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
Organocatalytic asymmetric deoxygenation of sulfones to access chiral sulfinyl compounds
The direct conversion of sulfones to chiral sulfinyl compounds is one of the major challenges in the fields of asymmetric synthesis and organosulfur chemistry. Now, through incorporation of a cyano group into the sulfone, an organocatalytic asymmetric deoxygenation strategy has been developed that enables the synthesis of chiral sulfinyl compounds.
- Shengli Huang
- , Zhen Zeng
- & Hailong Yan
-
Article |
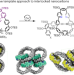 Active template strategy for the preparation of π-conjugated interlocked nanocarbons
Active template strategy for the preparation of π-conjugated interlocked nanocarbons
An active-template approach has been used to prepare π-conjugated interlocked nanocarbons derived from [n]cycloparaphenylenes. A metal ion bound within the central cavity of a precursor macrocycle first catalyses cross-coupling reactions and then the resulting mechanically interlocked intermediates are further transformed into π-conjugated species—[2] and [3]catenanes as well as a conjugated [3]rotaxane.
- James H. May
- , Jeff M. Van Raden
- & Ramesh Jasti
-
Article |
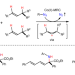 Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Metalloradical approach for concurrent control in intermolecular radical allylic C−H amination
Controlling various selectivities in radical reactions presents both formidable challenges and great opportunities. Now, Co(II)-based metalloradical catalysis has enabled the concurrent control of multiple convergences and selectivities in intermolecular radical allylic C−H amination. The reaction provides access to valuable chiral α-tertiary amines directly from an isomeric mixture of alkenes.
- Pan Xu
- , Jingjing Xie
- & X. Peter Zhang
-
Article
| Open Access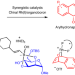 A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
Asymmetric systems for catalytic carbohydrate functionalization are mostly limited to chiral copper complexes and organocatalysts. Now, a synergistic chiral Rh(I)- and organoboron-catalysed site-selective functionalization of carbohydrate polyols has been developed, giving stereocontrolled access to biologically relevant arylhydronaphthalene glycosides. Enantio-, diastereo-, regio- and anomeric control and dynamic kinetic resolution were found to be concomitantly operative.
- V. U. Bhaskara Rao
- , Caiming Wang
- & Charles C. J. Loh
-
Article |
 Quantum interference effects elucidate triplet-pair formation dynamics in intramolecular singlet-fission molecules
Quantum interference effects elucidate triplet-pair formation dynamics in intramolecular singlet-fission molecules
Principles of quantum interference can guide the design of chromophores that undergo singlet fission. Now, ‘pencil and paper’ graphical models can be used to understand and predict the dynamics of triplet pairs generated through singlet fission in bridged dimers.
- Kaia R. Parenti
- , Rafi Chesler
- & Luis M. Campos
-
Article |
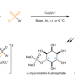 Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Cu-catalysed enantioselective radical heteroatomic S–O cross-coupling
Heteroatom–heteroatom cross-couplings have so far remained elusive. Now, a copper-catalysed enantioselective S–O cross-coupling of diverse diols or triols with sulfonyl chlorides has been realized via a single-electron reductive elimination manifold. The reaction provides access to value-added chiral C3 building blocks and inositol phosphates through enantioselective desymmetrization of biomass-derived alcohols.
- Yong-Feng Cheng
- , Zhang-Long Yu
- & Xin-Yuan Liu
-
News & Views |
 Radical arenes
Radical arenes
Radical-mediated functionalization streamlines access to complex synthetic targets. Now, a sulfonium-based donor–acceptor pair enables photoinduced charge-transfer interactions to access electronically diverse aryl radicals. Reaction with enol ethers or isocyanide provides a metal-free method for arene functionalization.
- Taylor N. Bednar
- & David A. Nagib
-
Article |
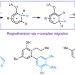 meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution
meta-Selective C–H arylation of phenols via regiodiversion of electrophilic aromatic substitution
The strong regiochemical preferences of electrophilic aromatic substitution have played a key role in defining the diversity of accessible chemical space. Now, it has been shown that the electrophilic arylation of phenols can be achieved at the electronically disfavoured meta-position via a formal 1,2-migration of a key σ-complex intermediate.
- Aaron Senior
- , Katie Ruffell
- & Liam T. Ball
-
Article |
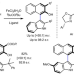 Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Enantioselective synthesis of atropisomeric indoles via iron-catalysed oxidative cross-coupling
Direct oxidative methods for the enantioselective synthesis of heterobiaryl compounds that exhibit axial chirality remain elusive. Now, the use of an iron catalyst in the presence of a chiral PyBOX ligand and an oxidant enables the direct coupling of naphthols and indoles with high levels of enantio- and cross-selectivity.
- Richard R. Surgenor
- , Xiangqian Liu
- & Martin D. Smith
-
Article |
 A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation
A general arene C–H functionalization strategy via electron donor–acceptor complex photoactivation
Photoactivation of EDA complexes was previously limited to electronically biased partners to secure productive charge-transfer interactions. Now, the participation of triarylsulfonium salts—formed by selective C–H sulfenylation—in photoactive EDA complexes with catalytic triarylamine donors provides a site-selective and metal-free strategy for the generation of aryl radicals and the formal C–H functionalization of native arenes.
- Abhishek Dewanji
- , Leendert van Dalsen
- & David J. Procter
-
Article |
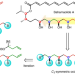 Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
Iterative synthesis of 1,3-polyboronic esters with high stereocontrol and application to the synthesis of bahamaolide A
Polyketide natural products often contain common repeat motifs that are synthesized using iterative processes. Now a masked 1,3-diol motif, generated by a two-step process based on boronic ester homologation, has enabled the efficient iterative synthesis of polyacetates, including bahamaolide A. In addition to oxidation, the 1,3-polyboronic esters were shown to undergo various stereospecific transformations.
- Sheenagh G. Aiken
- , Joseph M. Bateman
- & Varinder K. Aggarwal
-
Article |
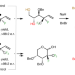 Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Diastereo- and enantioselective synthesis of compounds with a trifluoromethyl- and fluoro-substituted carbon centre
Methods to access organofluorine compounds with a trifluoromethyl- and fluoro-substituted carbon stereogenic centre are severely limited. Now, a flexible and stereodivergent approach has been developed for the efficient preparation of homoallylic alcohols containing this moiety. Conversion to polyfluoro furanosides and pyranosides has been demonstrated, which is relevant to antiviral drug development.
- Shibo Xu
- , Juan del Pozo
- & Amir H. Hoveyda
-
News & Views |
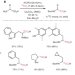 Enriched amino acids
Enriched amino acids
The direct carbon isotope exchange reaction on α-amino acids is highly desirable, as existing labelling methods require several synthetic steps and harsh conditions. Now, an aldehyde-catalysed carboxylate exchange with isotopically labelled *CO2 has enabled the direct formation of 11C, 13C and 14C-labelled α-amino acids.
- Karoline T. Neumann
- & Troels Skrydstrup
-
Article |
 Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2
Aldehyde-catalysed carboxylate exchange in α-amino acids with isotopically labelled CO2
Carbon-labelled α-amino acids are valuable compounds in drug development and nuclear medicine, but are difficult and time consuming to prepare. Now, an aldehyde-catalysed method has been developed for the direct C1-labelling of α-amino acids using *CO2 (* = 14, 13, 11), providing access to many proteinogenic and non-natural labelled α-amino acids.
- Odey Bsharat
- , Michael G. J. Doyle
- & Rylan J. Lundgren
-
Article |
 Stereocontrolled acyclic diene metathesis polymerization
Stereocontrolled acyclic diene metathesis polymerization
Polymerization methods that control the cis/trans stereochemistry of repeating alkenes in polyalkenamers remain scarce. Now, an acyclic diene metathesis process has been developed that enables control over the stereochemistry of the polymer backbone. The method harnesses the reactivity of dithiolate Ru carbenes, in combination with cis,cis-diene monomers, to access several classes of polymers with tailored properties.
- Ting-Wei Hsu
- , Samuel J. Kempel
- & Quentin Michaudel
-
Article
| Open Access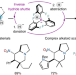 Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks
Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks
The rapid assembly of complex scaffolds in a single step from simple precursors would be an ideal reaction in terms of efficiency and sustainability. Now, the single-step construction of alkaloid-like frameworks from three dynamically assembled precursors has been reported. Using a dual-catalytic system, the transformation involves a hydride shuttle process initiated by a hydride donation event.
- Immo Klose
- , Giovanni Di Mauro
- & Nuno Maulide
-
Article |
 Targeted activation in localized protein environments via deep red photoredox catalysis
Targeted activation in localized protein environments via deep red photoredox catalysis
Technologies for profiling biological environments with high spatiotemporal resolution are in demand to enable the discovery of new targets for addressing unmet clinical needs. Now, a deep red light-mediated photocatalytic strategy for the targeted activation of aryl azides has been developed. This platform enables mapping of protein microenvironments in physiologically relevant systems.
- Nicholas Eng Soon Tay
- , Keun Ah Ryu
- & Tomislav Rovis
-
Article |
 An autonomous portable platform for universal chemical synthesis
An autonomous portable platform for universal chemical synthesis
Automated systems, nowadays more commonly used in laboratory settings, are typically fixed to a narrow set of reactions and used within a complex laboratory environment. Now, a portable platform has been developed for the on-demand and on-site multistep synthesis of organic molecules, oligonucleotides and oligopeptides mapped into reactionware systems.
- J. Sebastián Manzano
- , Wenduan Hou
- & Leroy Cronin
-
Article |
 Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Practical synthesis of the therapeutic leads tigilanol tiglate and its analogues
Tigilanol tiglate is a therapeutic lead for the treatment of a broad range of cancers. Now, it has been shown that tigilanol tiglate can be synthesized in a time and step economical fashion from phorbol—its naturally abundant biosynthetic precursor. This synthesis provides rapid access to analogues with unprecedented protein kinase C binding activity.
- Paul A. Wender
- , Zachary O. Gentry
- & Edward Njoo
-
News & Views |
 Isolating intermediates
Isolating intermediates
Noyori-type catalysts and inorganic bases are frequently used together for homogeneous hydrogenation, but key intermediates have not yet been isolated. Now, the structure and reactivity of a long-postulated intermediate — the alkali metal amidate complex — have been reported through experimental and computational studies.
- Pavel A. Dub
-
Article |
 Total synthesis of structurally diverse pleuromutilin antibiotics
Total synthesis of structurally diverse pleuromutilin antibiotics
General synthetic methods to access pleuromutilin antibiotics are limited due to their complex carbocyclic skeleton. Now, a synthetic platform has been developed to access structurally diverse pleuromutilins with variations at the quaternary C12 position and hydrindanone cores. Seventeen structurally distinct derivatives were prepared and evaluated against a panel of Gram-positive and -negative bacteria.
- Olivia Goethe
- , Mikaela DiBello
- & Seth B. Herzon
-
Article |
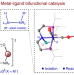 Structure, reactivity and catalytic properties of manganese-hydride amidate complexes
Structure, reactivity and catalytic properties of manganese-hydride amidate complexes
Noyori-type hydrogenation catalysts consist of an N–H moiety coordinated to a metal centre. Now, a metal-hydride amidate complex (HMn–NLi) has been isolated and found to have superior reactivity and catalytic performance compared with the corresponding HMn–NH complex, highlighting the superiority of M/NM′ bifunctional catalysis over the classic M/NH bifunctional catalysis for hydrogenation reactions.
- Yujie Wang
- , Shihan Liu
- & Qiang Liu
-
Article |
 Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
In silico chemical prediction of a polyketide synthase gene cluster in the bacterium Gynuella sunshinyii has led to the discovery of a class of natural products called janustatins. The absolute configuration of the stereocentres in these compounds was determined by a combination of techniques including DFT calculations and 2D NMR experiments—and finally confirmed by total synthesis. Janustatins were found to cause delayed, synchronized cell death at subnanomolar concentrations.
- Reiko Ueoka
- , Philipp Sondermann
- & Jörn Piel
-
Article |
 Scalable and continuous access to pure cyclic polymers enabled by ‘quarantined’ heterogeneous catalysts
Scalable and continuous access to pure cyclic polymers enabled by ‘quarantined’ heterogeneous catalysts
Cyclic polymers are topologically interesting and envisioned as a lubricant material, but methods for the scalable synthesis of pure cyclic polymers are currently elusive. Now, a scalable process has been developed by leveraging heterogeneity of the catalysts with the help of compartmentalized custom glassware, namely, a cyclic polymer dispenser.
- Ki-Young Yoon
- , Jinkyung Noh
- & Robert H. Grubbs
-
News & Views |
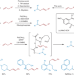 Back to basics
Back to basics
Amines with free N–H groups have long posed a tremendous challenge in transition metal-catalysed amination reactions. Now, use of a bidentate phosphorus ligand enables the palladium-catalysed oxidative amination of simple olefins with Lewis basic amines, with no prefunctionalization, forming both alkyl and aryl allylamines.
- Logan E. Vine
- & Jennifer M. Schomaker
-
Article |
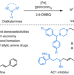 Palladium-catalysed selective oxidative amination of olefins with Lewis basic amines
Palladium-catalysed selective oxidative amination of olefins with Lewis basic amines
Given the importance of amine compounds, methods for their synthesis continue to be in high demand. Now, a palladium-catalysed strategy has been developed for the selective oxidative amination of unactivated olefins with Lewis basic amines, via C(sp3)–H activation, forming architecturally versatile and functionally diverse allylamines in a single step.
- Yangbin Jin
- , Yaru Jing
- & Huanfeng Jiang
-
Article |
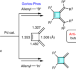 Palladium-catalysed construction of butafulvenes
Palladium-catalysed construction of butafulvenes
Butafulvene is a constitutional isomer of benzene, comprising a cyclobutene skeleton bearing two exocyclic conjugated methylene units. Strategies for the synthesis of butafulvene compounds are currently limited due to its intrinsic high strain energy and anti-aromaticity. Now, palladium-catalysed couplings have been developed for the rapid assembly of symmetric and non-symmetric anti-aromatic butafulvene derivatives.
- Xin Huang
- , Bing-Zhi Chen
- & Shengming Ma
-
Article |
 Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes
Photochemical single-step synthesis of β-amino acid derivatives from alkenes and (hetero)arenes
The majority of methods to prepare β-amino acid derivatives require metal-mediated multistep manipulations of pre-functionalized substrates. Now, a metal-free, energy-transfer enabled, highly regioselective aminocarboxylation reaction has been developed, for the single-step installation of both amine and ester functionalities into alkenes or (hetero)arenes. An oxime oxalate ester is used as a bifunctional reagent, supplying C-centred ester and N-centred iminyl radicals.
- Guangying Tan
- , Mowpriya Das
- & Frank Glorius
-
News & Views |
 Radical ring formation
Radical ring formation
N-heterocycles are valuable motifs in pharmaceuticals and materials, but divergent synthetic strategies are lacking. Now, bifunctional sulfilimines have been shown to form nitrogen-centred radicals under photocatalytic conditions, and subsequent coupling with olefins enables the synthesis of diverse N-heterocycles.
- Prabagar Baskaran
- & Wei Li
-
Article
| Open Access Bifunctional sulfilimines enable synthesis of multiple N-heterocycles from alkenes
Bifunctional sulfilimines enable synthesis of multiple N-heterocycles from alkenes
Intermolecular cyclization reactions using nitrogen-containing building blocks are scarce. Now, bifunctional sulfilimines have been shown to enable the modular construction of a diverse range of N-heterocycles by reacting with alkenes in a single photocatalysed step. Both sulfilimines and alkenes are easily accessible, providing access to a wide range of N-heterocycles with different ring types, ring sizes and substituents on the skeleton.
- Qiang Cheng
- , Zibo Bai
- & Tobias Ritter
-
News & Views |
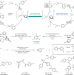 N2O revalorization
N2O revalorization
Nitrous oxide is traditionally considered to be an inert molecule, and methods for its activation and utilization are currently limited. Now, a strategy has been developed — involving an organometallic Baeyer–Villiger step — for the conversion of aryl halides to phenols under mild conditions, using N2O as the oxygen source.
- Jun-Jie Chen
- & Huan-Ming Huang
-
Article |
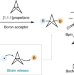 Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane
Exploiting the sp2 character of bicyclo[1.1.1]pentyl radicals in the transition-metal-free multi-component difunctionalization of [1.1.1]propellane
Methods to access bicyclo[1.1.1]pentane building blocks are limited, with current routes requiring multiple steps. Now, a diverse array of bicyclo[1.1.1]pentane boronates can be accessed via a multi-component reaction in a single step. Alkyl, aryl and alkenyl substructures can be installed onto bicyclo[1.1.1]pentane boronates by the use of carboxylic acids and organohalides.
- Weizhe Dong
- , Expédite Yen-Pon
- & Gary A. Molander
-
News & Views |
 Baird’s rules at the tipping point
Baird’s rules at the tipping point
This year marks the 50th anniversary of Baird’s rules of aromaticity — a set of perturbational molecular orbital theory analyses that has garnered considerable attention in the past ten years in light of its many real-world applications in photochemistry.
- Lucas J. Karas
- & Judy I. Wu
-
Article |
 Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Mechanically axially chiral catenanes and noncanonical mechanically axially chiral rotaxanes
Despite mechanically axially chiral (MAC) catenanes being recognized in 1961, their stereoselective synthesis had not been disclosed until now. Closer inspection of the MAC stereogenic unit has also led to the identification of an analogous, but unremarked upon, form of rotaxane stereochemistry and the conceptualization of a general approach to prepare MAC molecules stereoselectively.
- John R. J. Maynard
- , Peter Gallagher
- & Stephen M. Goldup
-
Article |
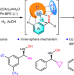 Nickel-catalysed asymmetric hydrogenation of oximes
Nickel-catalysed asymmetric hydrogenation of oximes
The asymmetric hydrogenation of oximes to chiral hydroxylamines is a long-standing challenge because of the labile N–O bond and inert C=N bond. Now, it has been shown that this reaction can be catalysed with a chiral nickel complex, and the weak interactions between catalyst and substrate are found to play a crucial role.
- Bowen Li
- , Jianzhong Chen
- & Wanbin Zhang
-
Article |
 Taming phosphorus mononitride
Taming phosphorus mononitride
Precursors for the release of phosphorus mononitride in solution under mild conditions have remained elusive. Now, an explosive anthracene-stabilized azidophosphine has shown PN transfer reactivity in the synthesis of an Fe–NP complex. The PN ligand is N-bonded, as the Fe–N interaction shows significant covalent character and a less unfavourable Pauli repulsion than its Fe–P counterpart.
- André K. Eckhardt
- , Martin-Louis Y. Riu
- & Christopher C. Cummins
-
Article |
 A multi-stage single photochrome system for controlled photoswitching responses
A multi-stage single photochrome system for controlled photoswitching responses
Moving beyond binary responses in photoswitches necessitates the introduction of new elements along their switching pathway. Now, a modified donor–acceptor Stenhouse adduct (DASA) capable of independently addressing multiple molecular states has been developed. This enables the transformation of a three-stage photoswitch to be controlled by regulating intermediates along the DASA reaction pathway.
- Friedrich Stricker
- , David M. Sanchez
- & Javier Read de Alaniz
-
News & Views |
 Complex networks at life’s origins
Complex networks at life’s origins
Explaining the controlled emergence and growth of molecular complexity at life’s origins is one of prebiotic chemistry’s grand challenges. Now, it has been shown that we can observe how the self-organization of a complex carbohydrate network can be modulated by its environment.
- Quentin Dherbassy
- & Kamila B. Muchowska
-
Article |
 Low-valent tungsten redox catalysis enables controlled isomerization and carbonylative functionalization of alkenes
Low-valent tungsten redox catalysis enables controlled isomerization and carbonylative functionalization of alkenes
The regioselectivity of tandem isomerization/hydrocarbonylation reactions is typically dictated by thermodynamics and there are limitations on the isomerization of internal alkenes. Now, it has been shown that a low-valent-tungsten catalyst controls the isomerization of alkenes to classically challenging unactivated internal positions and, with the aid of a directing group, enables subsequent addition of hydrogen and carbon monoxide.
- Tanner C. Jankins
- , William C. Bell
- & Keary M. Engle
-
Article |
 Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes
A general copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of diverse racemic tertiary alkyl halides with terminal alkynes has been developed, forging all-carbon quaternary stereocentres. Key to the success is the rational design of chiral anionic N,N,N-ligands tailor-made for the computationally predicted outer-sphere radical group transfer pathway.
- Fu-Li Wang
- , Chang-Jiang Yang
- & Xin-Yuan Liu
-
Article |
 E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing
E- and Z-trisubstituted macrocyclic alkenes for natural product synthesis and skeletal editing
Many bioactive compounds are trisubstituted macrocyclic alkenes, but use of current methods often results in poor yields and low stereoselectivity. Now, a ring-closing metathesis strategy has been developed that enables these compounds to be prepared efficiently and in either stereoisomeric form: an approach that may prove useful in the late stages of total syntheses, for skeletal editing and in drug discovery.
- Yucheng Mu
- , Felix W. W. Hartrampf
- & Amir H. Hoveyda
-
In Your Element |
 Bewildering benzene
Bewildering benzene
Claire Murray ponders on the attraction benzene — a small, seemingly simple molecule — has long exerted on scientists, some of the insights gained through its exploration, and the varied applications found for this hexagonal ring and its derivatives.
- Claire Murray

 Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres
Alkyl sulfinates as cross-coupling partners for programmable and stereospecific installation of C(sp3) bioisosteres