Article
|
Open Access
Featured
-
-
Article
| Open Access Bistability between π-diradical open-shell and closed-shell states in indeno[1,2-a]fluorene
Bistability between π-diradical open-shell and closed-shell states in indeno[1,2-a]fluorene
Switching the magnetic state of a polycyclic conjugated hydrocarbon in a reversible and controlled manner is challenging. Now, by means of single-molecule scanning probe microscopy, an indenofluorene isomer on ultrathin NaCl films has been shown to adopt both open- and closed-shell states. Furthermore, bidirectional switching between the two states is achieved by changing the adsorption site of the molecule.
- Shantanu Mishra
- , Manuel Vilas-Varela
- & Leo Gross
-
Article |
 Structural characterization and reactivity of a room-temperature-stable, antiaromatic cyclopentadienyl cation salt
Structural characterization and reactivity of a room-temperature-stable, antiaromatic cyclopentadienyl cation salt
The synthesis, structure and reactivity of room-temperature-stable [Cp(C6F5)5]+[Sb3F16]− is presented. Coordination of the cyclopentadienyl cation by [Sb3F16]− or C6F6 stabilizes the antiaromatic singlet state in the solid state. Calculated hydride and fluoride ion affinities of the [Cp(C6F5)5]+ cation are higher than those of the tritylium cation [C(C6F5)3]+.
- Yannick Schulte
- , Christoph Wölper
- & Stephan Schulz
-
News & Views |
 An open-shell molecule that exhibits conformational dynamics
An open-shell molecule that exhibits conformational dynamics
Open-shell organic molecules with properties that can be modulated by external stimuli are of interest for spintronics applications. Now, an overcrowded alkene with open-shell tetraradical character has been synthesized in which the interaction between the π-conjugated subunits depends on the charge and spin state.
- Yoshito Tobe
-
Article
| Open Access Tetrafluorenofulvalene as a sterically frustrated open-shell alkene
Tetrafluorenofulvalene as a sterically frustrated open-shell alkene
Tetrafluorenofulvalene (TFF) defies conventional rules of bond strength in organic chemistry. In particular, the central alkene bond of TFF becomes stronger in the quintet state and in the tetraanion. These changes arise from the unusual interplay between the twist, aromaticity and spin pairing in the π-electron system of TFF.
- Bibek Prajapati
- , Madan D. Ambhore
- & Marcin Stępień
-
Article |
 Time-resolved imaging and analysis of the electron beam-induced formation of an open-cage metallo-azafullerene
Time-resolved imaging and analysis of the electron beam-induced formation of an open-cage metallo-azafullerene
Visualizing single-molecule reactions using electron microscopy can be difficult because of potential radiation damage from the electron beam. Now, however, it has been shown that a high-energy electron beam can be used to synthesize metallo-azafullerenes. Atomic-resolution, time-resolved transmission electron microscopy, with the help of computational calculations, is used to monitor the metal-encapsulation dynamics.
- Helen Hoelzel
- , Sol Lee
- & Dominik Lungerich
-
Article
| Open Access Structural resolution of a small organic molecule by serial X-ray free-electron laser and electron crystallography
Structural resolution of a small organic molecule by serial X-ray free-electron laser and electron crystallography
The structural analysis of small crystals has remained challenging. Now, the structure of a small organic molecule, rhodamine-6G, has been resolved from microcrystals using an X-ray free-electron laser and electron diffraction. The former showed better reliability for atomic coordinates, whereas the latter was more sensitive to charges; both techniques accurately determined the position of hydrogen atoms.
- Kiyofumi Takaba
- , Saori Maki-Yonekura
- & Koji Yonekura
-
Article
| Open Access Rupturing aromaticity by periphery overcrowding
Rupturing aromaticity by periphery overcrowding
Stabilization from aromatic electron delocalization is highly favourable so it is typically preserved in even grossly distorted molecules. Now, peripheral overcrowding of an aromatic tropylium has been shown to cause sufficient geometric strain to rupture aromaticity, forming a non-aromatic bicyclic system that is in rapid equilibrium with its aromatic counterpart.
- Promeet K. Saha
- , Abhijit Mallick
- & Paul R. McGonigal
-
Article |
 Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
Genome-based discovery and total synthesis of janustatins, potent cytotoxins from a plant-associated bacterium
In silico chemical prediction of a polyketide synthase gene cluster in the bacterium Gynuella sunshinyii has led to the discovery of a class of natural products called janustatins. The absolute configuration of the stereocentres in these compounds was determined by a combination of techniques including DFT calculations and 2D NMR experiments—and finally confirmed by total synthesis. Janustatins were found to cause delayed, synchronized cell death at subnanomolar concentrations.
- Reiko Ueoka
- , Philipp Sondermann
- & Jörn Piel
-
In Your Element |
 Bewildering benzene
Bewildering benzene
Claire Murray ponders on the attraction benzene — a small, seemingly simple molecule — has long exerted on scientists, some of the insights gained through its exploration, and the varied applications found for this hexagonal ring and its derivatives.
- Claire Murray
-
News & Views |
 A hydroxide lock for metallo-β-lactamases
A hydroxide lock for metallo-β-lactamases
It is extremely difficult to design a broad-spectrum inhibitor for metallo-β-lactamases (MBLs) due to the diversity in the active site. Now, indole-2-carboxylates have been developed as broad-spectrum inhibitors for MBLs. These inhibitors take advantage of key elements of both MBL substrates and products and work by locking a hydroxide.
- Hongyan Li
- & Hongzhe Sun
-
Article |
 A thiolate-bridged FeIVFeIV μ-nitrido complex and its hydrogenation reactivity toward ammonia formation
A thiolate-bridged FeIVFeIV μ-nitrido complex and its hydrogenation reactivity toward ammonia formation
Multi-iron nitrides are implicated as potential key intermediates in biological nitrogen fixation and the industrial Haber–Bosch process, but well-described functional model systems are rare. Now, a well-defined thiolate-bridged FeIVFeIV μ-nitrido complex has been found to show excellent reactivity toward hydrogenation with H2 through a stepwise pathway to form ammonia in high yield.
- Yixin Zhang
- , Jinfeng Zhao
- & Jingping Qu
-
News & Views |
 More details on diazoolefins
More details on diazoolefins
Diazoolefins are long-sought-after compounds, but experimental evidence for them is limited because of their high reactivity. Now, it has been shown that the reaction of N-heterocyclic olefins with nitrous oxide provides access to diazoolefins, enabling structural characterization and applications in organometallic and synthetic chemistry.
- Claire Empel
- & Rene M. Koenigs
-
Article |
 Isolation and characterization of diazoolefins
Isolation and characterization of diazoolefins
Diazoolefins tend to be highly reactive compounds, and thus experimental evidence of these species is currently limited. Now, the reactivity and coordination chemistry of N-heterocyclic diazoolefins has been described. Diazoolefins are observed to form in reactions of N-heterocyclic olefins with nitrous oxide. The products benefit from resonance stabilization, enabling isolation on a preparative scale and comprehensive characterization.
- Paul Varava
- , Zhaowen Dong
- & Kay Severin
-
News & Views |
 Tectonic shifts in framework chemistry
Tectonic shifts in framework chemistry
Thirty years ago the assembly of molecular ‘tectons’ into organic networks with large chambers using directional non-covalent interactions — hydrogen bonds — provided a blueprint for the synthesis of porous functional materials through crystal engineering.
- Andrew I. Cooper
-
Article |
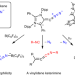 Isolation and reactivity of an elusive diazoalkene
Isolation and reactivity of an elusive diazoalkene
Although diazoalkenes have been reported as reactive intermediates in organic chemistry, their detection and isolation remains challenging. Such species have previously only been detected at low temperatures in matrix-isolation studies. Now, a room-temperature stable diazoalkene has been reported, which shows dual-site nucleophilicity and can undergo N2 exchange or lose dinitrogen under irradiation.
- P. W. Antoni
- , C. Golz
- & M. M. Hansmann
-
Article |
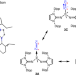 Isolable dicarbon stabilized by a single phosphine ligand
Isolable dicarbon stabilized by a single phosphine ligand
Diatomic C2 is an elusive species that has only been indirectly observed in the gas phase. It had previously been stabilized in the condensed phase using two ligands, but now a monoligated L→C2 complex has been prepared with a bulky phosphine ligand (L) bearing two imidazolidin-2-iminato groups. Reactivity studies and theoretical quantum chemical analysis point to the C2 moiety having a dicarbene character.
- Tsz-Fai Leung
- , Dandan Jiang
- & Gernot Frenking
-
Article |
 Emergence of low-symmetry foldamers from single monomers
Emergence of low-symmetry foldamers from single monomers
The majority of discrete structures obtained by self-assembly possess high symmetry, and thus low complexity: all subunits relate to their neighbours in a similar manner. Now, the spontaneous formation of complex low-symmetry assemblies produced from a single building block has been demonstrated using a systems chemistry approach. The single building block oligomerizes to form specific homomeric cyclic macromolecules that adopt a folded conformation.
- Charalampos G. Pappas
- , Pradeep K. Mandal
- & Sijbren Otto
-
Article |
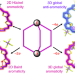 3D global aromaticity in a fully conjugated diradicaloid cage at different oxidation states
3D global aromaticity in a fully conjugated diradicaloid cage at different oxidation states
A conjugated diradicaloid cage has been synthesized and its aromaticity was investigated. The neutral compound and the dication have dominant monocyclic conjugation pathways and both are aromatic (the former following Hückel’s rule and the latter Baird’s rule). The tetracation ([6n + 4] π-electrons) exhibits global 3D antiaromaticity whereas the hexacation ([6n + 2] π-electrons) exhibits global 3D aromaticity and has high D3 symmetry.
- Yong Ni
- , Tullimilli Y. Gopalakrishna
- & Jishan Wu
-
Article |
 Synthesis and reactivity of precolibactin 886
Synthesis and reactivity of precolibactin 886
Precolibactin 886 is a complex microbiome-derived metabolite implicated in colorectal cancer and produced by the clb gene cluster. A chemical synthesis and analysis of precolibactin 886 is reported which shows that its biosynthetic precursor degrades to other known clb metabolites. The data also provide insights into the structures and reactivity of advanced clb products.
- Alan R. Healy
- , Kevin M. Wernke
- & Seth B. Herzon
-
Article |
 Identification of autoinducing thiodepsipeptides from staphylococci enabled by native chemical ligation
Identification of autoinducing thiodepsipeptides from staphylococci enabled by native chemical ligation
Bacteria communicate through excretion of minute amounts of chemical signalling molecules that affect virulence, biofilm formation and colonization. In staphylococci, these molecules, called autoinducing peptides, are macrocyclic thiolactone-containing peptides. Now, a simple enrichment method, based on chemoselective capture on polymer beads, has been developed that enables the identification of previously unknown autoinducing peptides.
- Bengt H. Gless
- , Martin S. Bojer
- & Christian A. Olsen
-
News & Views |
 Big insights from tiny crystals
Big insights from tiny crystals
Most compounds form crystals so small that scientists cannot experimentally determine their atomic structures using X-ray crystallography. Microcrystal electron diffraction now provides a unique solution for this challenge.
- Oleg Sitsel
- & Stefan Raunser
-
Article |
 Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets
Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets
A paradoxical case of a well-defined diradicaloid that has an unusually large singlet–triplet energy gap (ΔES-T) imparted by the thiophene sulfur atom is reported. Quantum chemistry, organic synthesis, molecular spectroscopies, X-ray crystal analysis and high-temperature magnetic measurements help account for the dichotomy between the large diradical character and large ΔEST.
- Justin J. Dressler
- , Mitsuru Teraoka
- & Michael M. Haley
-
Article |
 Ribosomal synthesis and folding of peptide-helical aromatic foldamer hybrids
Ribosomal synthesis and folding of peptide-helical aromatic foldamer hybrids
The extent to which peptide synthesis by the ribosome can tolerate the inclusion of non-peptidic components is not clear. Yet such hybrids would expand the range of ribosomally synthesized structures. Now it has been shown that tRNAs acylated by aromatic foldamers can initiate the ribosomal synthesis of non-cyclic and cyclic foldamer–peptide hybrid molecules. The oligo-aryl segments contain folding information that can control peptide conformation in the hybrids.
- Joseph M. Rogers
- , Sunbum Kwon
- & Ivan Huc
-
Article |
 Catching elusive glycosyl cations in a condensed phase with HF/SbF5 superacid
Catching elusive glycosyl cations in a condensed phase with HF/SbF5 superacid
Glycosyl cations are universally accepted as key intermediates in the mechanism of glycosylation—the reaction that covalently links carbohydrates to other molecules—but their high reactivity makes them difficult to characterize. Using HF/SbF5 superacid, two glucosyl cations have been generated and stabilized, then characterized by NMR spectroscopy aided by computation and their conformation elucidated.
- A. Martin
- , A. Arda
- & Y. Blériot
-
-
-
-
Article |
 Organic structure determination using atomic-resolution scanning probe microscopy
Organic structure determination using atomic-resolution scanning probe microscopy
The structure of many natural products can often only be confirmed by comparison with a synthetic sample. Here, scanning probe microscopy techniques allow the ultimate discrimination between structures suggested by the standard range of analytical techniques, proving the power of single-molecule imaging for molecular structure determination.
- Leo Gross
- , Fabian Mohn
- & Marcel Jaspars
-
Research Highlights |
 Structure of a strained ring
Structure of a strained ring
Dimethylcyclobutadiene confined in a crystalline network can be structurally characterized by X-ray crystallography.
- Anne Pichon
-
Article |
 Random two-dimensional string networks based on divergent coordination assembly
Random two-dimensional string networks based on divergent coordination assembly
The bulk properties of materials that lack long-range order have been widely studied, but their local structures remain difficult to elucidate. Now, using scanning tunnelling microscopy, researchers have been able to look more closely at the structural motifs of robust, two-dimensional glassy networks assembled through metal–ligand interactions.
- Matthias Marschall
- , Joachim Reichert
- & Johannes V. Barth

 The anti-aromatic dianion and aromatic tetraanion of [18]annulene
The anti-aromatic dianion and aromatic tetraanion of [18]annulene Non-classical crystals
Non-classical crystals Anti-anthrax agents
Anti-anthrax agents Structural snapshots
Structural snapshots