Abstract
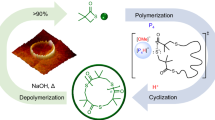
Cyclic polymers are topologically interesting and envisioned as a lubricant material. However, scalable synthesis of pure cyclic polymers remains elusive. The most straightforward way is to recover a used catalyst after the synthesis of cyclic polymers and reuse it. Unfortunately, this is demanding because of the catalyst’s vulnerability and inseparability from polymers, which reduce the practicality of the process. Here we develop a continuous circular process, where polymerization, polymer separation and catalyst recovery happen in situ, to dispense a pure cyclic polymer after bulk ring-expansion metathesis polymerization of cyclopentene. It is enabled by introducing silica-supported ruthenium catalysts and newly designed glassware. Different depolymerization kinetics of the cyclic polymer from its linear analogue are also discussed. This process minimizes manual labour, maximizes the security of vulnerable catalysts and guarantees the purity of cyclic polymers, thereby showcasing a prototype of a scalable access to cyclic polymers with increased turnovers (≥415,000) of precious catalysts.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
Change history
21 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41557-022-01066-0
References
González-Reyes, G. A., Bayo-Besteiro, S., Vich Llobet, J. & Añel, J. A. Environmental and economic constraints on the use of lubricant oils for wind and hydropower generation: the case of NATURGY. Sustainability 12, 4242 (2020).
Wakiru, J., Pintelon, L., Muchiri, P. N., Chemweno, P. K. & Mburu, S. Towards an innovative lubricant condition monitoring strategy for maintenance of ageing multi-unit systems. Reliab. Eng. Syst. 204, 107200 (2020).
Zolper, T. et al. Lubrication properties of polyalphaolefin and polysiloxane lubricants: molecular structure–tribology relationships. Tribol. Lett. 48, 355–365 (2012).
Greaves, M. Pressure viscosity coefficients and traction properties of synthetic lubricants for wind turbine gear systems. Lubr. Sci. 24, 75–83 (2012).
Ray, S., Rao, P. V. C. & Choudary, N. V. Poly-α-olefin-based synthetic lubricants: a short review on various synthetic routes. Lubr. Sci. 24, 23–44 (2012).
Martini, A., Ramasamy, U. S. & Len, M. Review of viscosity modifier lubricant additives. Tribol. Lett. 66, 58 (2018).
Morgan, S., Ye, Z., Subramanian, R. & Zhu, S. Higher-molecular-weight hyperbranched polyethylenes containing crosslinking structures as lubricant viscosity-index improvers. Polym. Eng. Sci. 50, 911–918 (2010).
Ver Strate, G. & Struglinski, M. J. in Polymers as Rheology Modifiers, ACS Symposium Series Vol. 462 (eds Schulz, D. N. & Glass, J. E.) Ch. 15 (American Chemical Society, 1991).
Peterson, G. I. & Choi, T.-L. The influence of polymer architecture in polymer mechanochemistry. Chem. Commun. 57, 6465–6474 (2021).
Lin, Y., Zhang, Y., Wang, Z. & Craig, S. L. Dynamic memory effects in the mechanochemistry of cyclic polymers. J. Am. Chem. Soc. 141, 10943–10947 (2019).
Bielawski, C. W., Benitez, D. & Grubbs, R. H. An ‘endless’ route to cyclic polymers. Science 297, 2041–2044 (2002).
Xia, Y. et al. Ring-expansion metathesis polymerization: catalyst-dependent polymerization profiles. J. Am. Chem. Soc. 131, 2670–2677 (2009).
Boydston, A. J., Xia, Y., Kornfield, J. A., Gorodetskaya, I. A. & Grubbs, R. H. Cyclic ruthenium-alkylidene catalysts for ring-expansion metathesis polymerization. J. Am. Chem. Soc. 130, 12775–12782 (2008).
Xia, Y., Boydston, A. J. & Grubbs, R. H. Synthesis and direct imaging of ultrahigh molecular weight cyclic brush polymers. Angew. Chem. Int. Ed. 50, 5882–5885 (2011).
Boydston, A. J., Holcombe, T. W., Unruh, D. A., Fréchet, J. M. J. & Grubbs, R. H. A direct route to cyclic organic nanostructures via ring-expansion metathesis polymerization of a dendronized macromonomer. J. Am. Chem. Soc. 131, 5388–5389 (2009).
Bielawski, C. W., Benitez, D. & Grubbs, R. H. Synthesis of cyclic polybutadiene via ring-opening metathesis polymerization: the importance of removing trace linear contaminants. J. Am. Chem. Soc. 125, 8424–8425 (2003).
Wang, T.-W., Huang, P.-R., Chow, J. L., Kaminsky, W. & Golder, M. R. A cyclic ruthenium benzylidene initiator platform enhances reactivity for ring-expansion metathesis polymerization. J. Am. Chem. Soc. 143, 7314–7319 (2021).
Miao, Z. et al. Cyclic polyacetylene. Nat. Chem. 13, 792–799 (2021).
McGraw, M. L., Clarke, R. W. & Chen, E. Y. X. Synchronous control of chain length/sequence/topology for precision synthesis of cyclic block copolymers from monomer mixtures. J. Am. Chem. Soc. 143, 3318–3322 (2021).
Niu, W. et al. Polypropylene: now available without chain ends. Chem 5, 237–244 (2019).
Roland, C. D., Li, H., Abboud, K. A., Wagener, K. B. & Veige, A. S. Cyclic polymers from alkynes. Nat. Chem. 8, 791–796 (2016).
Lidster, B. J. et al. Macrocyclic poly(p-phenylenevinylene)s by ring expansion metathesis polymerisation and their characterisation by single-molecule spectroscopy. Chem. Sci. 9, 2934–2941 (2018).
Zhang, K., Lackey, M. A., Wu, Y. & Tew, G. N. Universal cyclic polymer templates. J. Am. Chem. Soc. 133, 6906–6909 (2011).
Edwards, J. P., Wolf, W. J. & Grubbs, R. H. The synthesis of cyclic polymers by olefin metathesis: achievements and challenges. J. Polym. Sci. A Polym. Chem. 57, 228–242 (2018).
Chang, Y. A. & Waymouth, R. M. Recent progress on the synthesis of cyclic polymers via ring-expansion strategies. J. Polym. Sci. A Polym. Chem. 55, 2892–2902 (2017).
Haque, F. M. & Grayson, S. M. The synthesis, properties and potential applications of cyclic polymers. Nat. Chem. 12, 433–444 (2020).
Golba, B., Benetti, E. M. & De Geest, B. G. Biomaterials applications of cyclic polymers. Biomaterials 267, 120468 (2021).
Miao, Z. et al. Semi-conducting cyclic copolymers of acetylene and propyne. React. Funct. Polym. 169, 105088 (2021).
Tuba, R. Synthesis of cyclopolyolefins via ruthenium catalyzed ring-expansion metathesis polymerization. Pure Appl. Chem. 86, 1685–1693 (2014).
Jawiczuk, M., Marczyk, A. & Trzaskowski, B. Decomposition of ruthenium olefin metathesis catalyst. Catalysts 10, 887 (2020).
Allen, D. P., Van Wingerden, M. M. & Grubbs, R. H. Well-defined silica-supported olefin metathesis catalysts. Org. Lett. 11, 1261–1264 (2009).
Dewaele, A., Van Berlo, B., Dijkmans, J., Jacobs, P. A. & Sels, B. F. Immobilized Grubbs catalysts on mesoporous silica materials: insight into support characteristics and their impact on catalytic activity and product selectivity. Catal. Sci. Technol. 6, 2580–2597 (2016).
Hejl, A., Scherman, O. A. & Grubbs, R. H. Ring-opening metathesis polymerization of functionalized low-strain monomers with ruthenium-based catalysts. Macromolecules 38, 7214–7218 (2005).
Neary, W. J. & Kennemur, J. G. Polypentenamer renaissance: challenges and opportunities. ACS Macro Lett. 8, 46–56 (2019).
Tuba, R. & Grubbs, R. H. Ruthenium catalyzed equilibrium ring-opening metathesis polymerization of cyclopentene. Polym. Chem. 4, 3959–3962 (2013).
Neary, W. J. & Kennemur, J. G. Variable temperature ROMP: leveraging low ring strain thermodynamics to achieve well-defined polypentenamers. Macromolecules 50, 4935–4941 (2017).
Mulhearn, W. D. & Register, R. A. Synthesis of narrow-distribution, high-molecular-weight ROMP polycyclopentene via suppression of acyclic metathesis side reactions. ACS Macro Lett. 6, 112–116 (2017).
Lee, L.-B. W. & Register, R. A. Acyclic metathesis during ring-opening metathesis polymerization of cyclopentene. Polymer 45, 6479–6485 (2004).
Ji, S., Hoye, T. R. & Macosko, C. W. Controlled synthesis of high molecular weight telechelic polybutadienes by ring-opening metathesis polymerization. Macromolecules 37, 5485–5489 (2004).
Obligacion, J. V. & Chirik, P. J. Bis(imino)pyridine cobalt-catalyzed alkene isomerization–hydroboration: a strategy for remote hydrofunctionalization with terminal selectivity. J. Am. Chem. Soc. 135, 19107–19110 (2013).
Ulman, M. & Grubbs, R. H. Ruthenium carbene-based olefin metathesis initiators: catalyst decomposition and longevity. J. Org. Chem. 64, 7202–7207 (1999).
Torre Iii, M., Mulhearn, W. D. & Register, R. A. Ring-opening metathesis copolymerization of cyclopentene above and below its equilibrium monomer concentration. Macromol. Chem. Phys. 219, 1800030 (2018).
Szczepaniak, G., Kosiński, K. & Grela, K. Towards ‘cleaner’ olefin metathesis: tailoring the NHC ligand of second generation ruthenium catalysts to afford auxiliary traits. Green Chem. 16, 4474–4492 (2014).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Neary, W. J., Isais, T. A. & Kennemur, J. G. Depolymerization of bottlebrush polypentenamers and their macromolecular metamorphosis. J. Am. Chem. Soc. 141, 14220–14229 (2019).
Yuan, J., Giardino, G. J. & Niu, J. Metathesis cascade-triggered depolymerization of enyne self-immolative polymers. Angew. Chem. Int. Ed. 60, 24800–24805 (2021).
Acknowledgements
R. H. Grubbs passed away on 19th December 2021 and was a corresponding author when the article was first submitted. This work is financially supported by the National Science Foundation (CHE#1807154) and the Creative Research Initiative Grant. N. Hart at Caltech Glass Shop is gratefully acknowledged for the glass blowing. S. Hwang at Caltech Solid State NMR Facility is thanked for the solid-state NMR. We thank NCIRF at Seoul National University for supporting headspace gas chromatography–mass spectrometry experiments. Y. Xu (Peking University), J. H. Ko (Caltech), J.-A. Song (Samsung), Y.-J. Jang (University of Minnesota) and D. Allen (Materia) are acknowledged for helpful discussions.
Author information
Authors and Affiliations
Contributions
R.H.G. and K.-Y.Y. conceived and designed the project. R.H.G. and T.-L.C. directed the project and provided valuable input. K.-Y.Y., Q.G. and J.P.E. synthesized the catalysts. K.-Y.Y. designed the glassware. K.-Y.Y. and Q.G. conducted polymer synthesis. K.-Y.Y., J.N. and Q.G. characterized the polymers. J.N. performed depolymerization experiments. R.T. demonstrated the heterogeneous cyclic polymer process. All authors analysed the data and discussed the results. K.-Y.Y. wrote the manuscript and then all authors reviewed and commented on it.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Matthew Golder, Farihah Haque and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–6 and Sections 1–18.
Source data
Source Data Fig. 3
Data for plots (Fig. 3c,d).
Source Data Fig. 4
Data for plots (Fig. 4c,d).
Source Data Fig. 5
Data for Fig. 5b.
Source Data Fig. 6
Data for Fig. 6a.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoon, KY., Noh, J., Gan, Q. et al. Scalable and continuous access to pure cyclic polymers enabled by ‘quarantined’ heterogeneous catalysts. Nat. Chem. 14, 1242–1248 (2022). https://doi.org/10.1038/s41557-022-01034-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-01034-8