Abstract
Chronic cellular stress has a profound impact on the brain, leading to degeneration and accelerated aging. Recent work has revealed the vital role of RNA modifications, and the proteins responsible for regulating them, in the stress response. In our study, we defined the role of CG14618/dTrmt10A, the Drosophila counterpart of human TRMT10A a N1-methylguanosine methyltransferase, on m6A regulation and heat stress resilience in the Drosophila brain. By m6A-IP RNA sequencing on Drosophila head tissue, we demonstrated that manipulating dTrmt10A levels indirectly regulates m6A levels on polyA + RNA. dTrmt10A exerted its influence on m6A levels on transcripts enriched for neuronal signaling and heat stress pathways, similar to the m6A methyltransferase Mettl3. Intriguingly, its impact primarily targeted 3' UTR m6A, setting it apart from the majority of Drosophila m6A-modified transcripts which display 5' UTR enrichment. Upregulation of dTrmt10A led to increased resilience to acute heat stress, decreased m6A modification on heat shock chaperones, and coincided with decreased decay of chaperone transcripts and increased translation of chaperone proteins. Overall, these findings establish a potential mechanism by which dTrmt10A regulates the acute brain stress response through m6A modification.
Similar content being viewed by others
Introduction
RNA modifications are emerging as a critical layer of gene expression that impact various biological functions in the brain, such as learning and memory, neurogenesis, and developmental transitions1,2,3,4,5,6,7. Mutations in methylation complex proteins and dysregulation in RNA modification can lead to severe developmental defects, compromised cellular stress responses, and tumorigenesis8,9,10. TRMT10A is an S-adenosylmethionine-dependent methyltransferase that installs the RNA modification N1-methylguanosine (m1G) on tRNAs at the ninth position; it is the only known methyltransferase that installs m1G on human tRNA11,12. tRNAs are among the most heavily modified classes of RNA and these modifications are critical for the stability and translation functionality of tRNAs11,12. Mutations of TRMT10A in humans have been linked to primary microcephaly, mild intellectual disability, young onset diabetes, and apoptosis in pancreatic cells13,14,15,16,17. However, the mechanisms by which genetic mutations in TRMT10A cause these phenotypes are not well understood.
N6-methyladenosine (m6A) is the most abundant internal modification of eukaryotic mRNA1,18,19. It is deposited co-transcriptionally by a methyltransferase, METTL3, with the help of additional structural proteins. m6A levels are dynamically regulated by writer, reader, and eraser proteins8,19. The m6A modification of mRNA provides a critical layer of regulation in neuronal responses to cellular stress and disease20,21,22. Interaction of m6A transcripts with reader proteins can dictate downstream processing, including splicing, decay, and translation23,24,25,26. TRMT10A has been shown to interact with the m6A RNA demethylase FTO and promote its demethylase activity in HEK293T cells27. Loss of TRMT10A increases m6A methylation on FTO RNA targets in cultured cells27. m6A levels are enriched in brain tissue of human, mice, and Drosophila, and mark transcripts involved in synaptic growth, axon guidance and signaling22,28,29,30. m6A reader proteins, or YTH domain-containing proteins, are known for their ability to promote translation and decay of m6A-modified RNAs7,31,32,33. One of their characterized functions is the ability to regulate cortical neurogenesis through precise decay of neuronal m6A transcripts during developmental transitions in mouse and human cells30,33.
During cellular stress, the role of m6A modifications becomes amplified with increased methylation dynamically regulating RNAs for rapid degradation and/or selective translation20,28,34,35,36,37. During UV DNA damage stress, m6A methylation functions in recruitment of DNA damage machinery38, and during heat stress transcripts become marked for decay and translation in in vitro human cell culture models39,40,41. Neurons are critically vulnerable to prolonged stress and degenerative disease. Heat shock protein chaperones (HSPs) are known to protect neuronal cells against protein misfolding, accumulation and aggregation, but HSPs are dysregulated during aging and disease42.
Drosophila is a powerful genetic model system that has pioneered numerous fundamental discoveries in brain development and disease. Homologous m6A methyltransferase complex components are enriched in the Drosophila central nervous system (CNS), making the fly an ideal system for understanding m6A modification in brain functions19. In Drosophila, m6A is implicated in female specific alternative splicing of the master sex determination regulatory gene Sex-lethal (Sxl)23,43,44. Additionally, the Drosophila cytoplasmic m6A reader protein Ythdf regulates axonal overgrowth through interactions with Fmr1, the fly homolog of Fragile X mental retardation protein, and together they inhibit translation of m6A modified targets of Fmr145. Furthermore, there is no identified homologous m6A demethylase in Drosophila.
Here, we investigate the fly homolog of TRMT10A, CG14618 (which we now refer to as dTrmt10A), to uncover its regulation of m6A levels and the stress response in the Drosophila brain. The role of TRMT10A/dTrmt10A and its effects on m6A levels have not been studied in vivo. Our m6A-IP sequencing results indicate that dTrmt10A impacts m6A on signaling transcripts, and alters m6A on key heat stress chaperone transcripts3,46. We find that upregulation of dTrmt10A acts similarly to loss of m6A methyltransferase Mettl3 in the Drosophila brain, and highlights m6A peaks in the 3’UTR of transcripts. These findings indicate that dTrmt10A modulates m6A in Drosophila and plays a critical role in regulating the stress response of the brain.
Results
m6A is enriched in the brain and dTrmt10A knockdown increases m6A levels on Hsp70 transcripts.
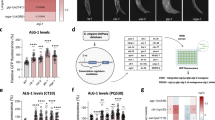
The Drosophila gene CG14618 is the predicted homolog of the human TRMT10A methyltransferase. Using Clustal Omega, human TRMT10A and Drosophila CG14618 share 36.8% amino acid sequence identity, with 100% conservation in the m1G catalytic domain "YVIGGLVDHNH" (Fig. 1A). We refer to CG14618 as Drosophila Trmt10A (dTrmt10A) from here on. Knockdown of dTrmt10A in vivo produced viable offspring. Using liquid chromatography-tandem mass spectrometry (LC–MS/MS), we confirmed significant m1G level reduction in dTrmt10A RNAi brains (small RNA fraction < 200 nucleotides), compared to control brains (Fig. 1B). dTrmt10A mRNA levels in head tissue were significantly reduced by RT-qPCR and RNA-seq analysis (Fig. 1C,D and Supplementary Data 1).
Impact of the Drosophila homolog of TRMT10A on m1G and m6A levels. (A) Alignment of Human TRMT10A and Drosophila dTrmt10A/CG14618 by Clustal Omega multiple sequence comparison. Asterisk * indicates fully conserved residues, a colon indicates conservation of strongly similar properties, and period indicates conservation of weakly similar properties. Protein alignment shows overall 36% conservation between Drosophila and human protein. Red line indicates conserved m1G catalytic domain, and G206R mutation highlighted in yellow indicates catalytic mutant. (B) LC–MS/MS analysis of m1G/G% levels from brain small RNA in basal conditions, in control vs dTrmt10A RNAi, n = 5 biological replicates, 30 brains per replicate. Data are presented as mean, t-test *p < 0.05. (C) RNA levels of dTrmt10A were assessed by RT-qPCR. daGal4 > UAS-KK control (Vienna 60100); daGal4 > UAS-dTrmt10A RNAi (Vienna 100720); n = 3 biological replicates, 15 brains per replicate. Data are presented as mean, t-test, ***p < 0.001. (D) RNA levels of dTrmt10A determined from RNA-sequencing Drosophila head tissue, normalized read counts and statistical analysis done by Deseq2. See Supplementary Data 1 for details of fly genotypes in all figures.
Given that TRMT10A interacts with FTO in mammalian cells27, we conducted m6A-IP sequencing analysis of polyA + RNA from Drosophila heads expressing dTrmt10A RNAi. IP sequencing confirmed that m6A is enriched in the 5'UTR of transcripts in Drosophila heads, consistent with our and others previous work3,46. Enrichment of m6A in the 5'UTR is unique to Drosophila, as mammalian m6A is primarily located in the 3'UTR28. Global metagene analysis highlighted that dTrmt10A knockdown increased m6A levels in the 5'UTR of transcripts, and unexpectedly also showed increased m6A in the 3'UTR of a subset of transcripts (Fig. 2A).
dTrmt10A knockdown increases m6A on the 5′UTR of Hsp70 transcripts. (A) Normalized read coverage plot of m6A-IP/input on polyA + transcripts in the 5′UTR, CDS, and 3′UTR of m6A genes. m6A-IP sequencing in basal conditions from fly heads (daGal4 > KK control vs daGal4 > dTrmt10A RNAi). (B) IGV genome browser tracks of m6A-IP and Input reads for Hsp70 genes in basal conditions, from control and dTrmt10A RNAi. Red box highlights increase of 5’UTR m6A with dTrmt10A RNAi. (C) RT-qPCR shows decreased levels of Hsp70 RNA in basal conditions from KK control and dTrmt10A RNAi brains. t-test, **p < 0.01. (D) dTrmt10A RNAi decreased protein levels of Hsp70 in basal and HS conditions. t-test, *p < 0.05. (E) daGal4 > KK control vs daGal4 > dTrmt10A RNAi and elavGal4 > KK control vs elavGal4 > dTrmt10A RNAi increased heat stress sensitivity of the animals. Animals heat stressed for 1 h at 38.5 °C and allowed to recover for 24 h. t-test, ***p < 0.001. **p < 0.01. (F) Scatterplot of normalized read coverage of m6A-IP/input for 5′UTR of m6A transcripts in control versus dTrmt10A RNAi conditions. dTrmt10A RNAi shows a shift towards increased m6A levels in 5’UTR. (G) Genome browser tracks of m6A-IP and Input reads for DnaJ-1 in basal conditions, from control and dTrmt10A RNAi. Red box highlights increased 5’UTR m6A upon dTrmt10A upregulation. RADAR differential peak analysis in Supplemental Data 2. (H) RNA decay assay from Control and dTrmt10A RNAi fly brains, dissected and incubated in Schneider’s Drosophila Medium plus 169.5ug/ml of actinomycin D for 4 h. RNA was extracted and used for RT-qPCR to determine relative RNA levels of RpL32, and Hsp70. Data are presented as mean ± SD of n = 4 biological replicates, 12 brains per replicate. **p < 0.01. Student’s two-tailed t-test. ns = not significant.
To determine which transcripts showed significant differential methylation between control and dTrmt10A knockdown conditions, we utilized RADAR analysis with a beta-threshold of 0.5. Using these parameters, only transcripts from a single gene showed a significant increase in m6A. These transcripts encode the heat shock chaperone protein Hsp70 (Fig. 2B). We were particularly intrigued by this finding as there are normally low levels of m6A in the 5'UTR on Hsp70 transcripts at baseline, and m6A signal is lost upon acute heat stress in the Drosophila brain46. Previously, we found that knockdown of m6A methyltransferase Mettl3 showed increased RNA and protein levels of Hsp70 at baseline along with increased heat stress resilience46. We asked if an opposite effect occurred with knockdown of dTrmt10A. In dTrmt10A RNAi brains, RNA and protein levels of Hsp70 were reduced (Fig. 2C,D). Additionally, dTrmt10A RNAi animals exhibited increased stress sensitivity (Fig. 2E). These data indicate that dTrmt10A RNAi exerts contrasting effects on Hsp70 RNA, protein, and m6A levels compared to Mettl3 RNAi.
To visualize global changes in m6A, regardless of peak calling threshold, we generated a scatterplot of m6A-IP reads divided by input read coverage in the 5'UTR for Mettl3-dependent m6A transcripts. Upon dTrmt10A knockdown, we observed a global shift towards increased levels of m6A in the 5'UTR of transcripts (Fig. 2F), similar to the global metagene analysis (Fig. 2A). We observed a slight increase of 5’UTR m6A on other chaperones such as Heat shock chaperone 40 (DnaJ-1) (Fig. 2G), but no changes comparable to the increase on Hsp70 5’UTR. These findings highlight the regulation of Hsp70 transcripts by the m6A machinery, and indicate it as key target of dTrmt10A.
To analyze the impact of m6A on RNA levels with dTrmt10A manipulation, we employed an ex-vivo brain RNA decay assay. Reduced m6A by Mettl3 RNAi lead to higher steady state levels and decreased rate of decay of m6A transcripts46. We examined the relative levels of RNA remaining in dTrmt10A RNAi brain samples after 4 h, in the presence of actinomycin D. dTrmt10A RNAi brains (with increased m6A) had increased decay of Hsp70 RNA transcript levels compared to control brains, and no significant difference of RpL32, which is a control non-m6A modified transcript (Fig. 2H). These data suggest that dTrmt10A influences the stability of Hsp70 transcripts and overall protein and RNA levels, likely by modulating their m6A levels.
Upregulation of dTrmt10A decreases m6A levels and enhances the stress response of the brain.
Studies investigating the role of TRMT10A in cultured cells have focused on mutations or gene downregulation17,27. To determine whether upregulation of dTrmt10A could also regulate m6A levels, we utilized a FLAG-HA C-terminal tagged ORF clone of dTrmt10A to generate animals that upregulate dTrmt10A (Supplementary Fig. 1A). Upregulation of dTrmt10A showed no global effect on m1G levels, likely due to the saturation of m1G levels on tRNAs (Supplementary Fig. 1B). m6A-IP sequencing analysis conducted on Drosophila heads with dTrmt10A upregulation revealed a striking reduction in m6A levels (Fig. 3A). A closer look at heat stress chaperones revealed 5’UTR m6A normally present at baseline on Hsp70 transcripts was lost upon dTrmt10A upregulation, conferring the opposite effect from dTrmt10A RNAi (Fig. 3B). Additionally, Hsp70 RNA and protein levels were elevated in UAS-dTrmt10A brains (Fig. 3C,D), and animals were more resilient to heat stress (Fig. 3E). Loss of Mettl3 also leads to elevated Hsp70 transcript and protein levels. To provide support that the change in Hsp70 with dTrmt10A upregulation was due to effects on m6A, vs other possibilities, we simultaneously reduced Mettl3 and upregulated dTrmt10A in neurons. Co-expression of Mettl3 RNAi and UAS-dTrmt10A in the brain showed a similar increase in Hsp70 protein levels compared to expression of either transgene alone (Fig. 3F). These data suggest that dTrmt10A is acting through the m6A/Mettl3 pathway to modulate Hsp70 levels.
dTrmt10A upregulation confers heat stress resilience. (A) Normalized read coverage plot of m6A-IP/input on polyA + transcripts in the 5’UTR, CDS, and 3’UTR of m6A modified transcripts. m6A-IP sequencing in basal conditions from control fly (daGal4 > KK control) versus dTrmt10A upregulation (daGal4 > UAS-dTrmt10A) heads. (B) IGV genome browser tracks of m6A-IP enrichment versus Input reads for Hsp70 in basal conditions, from control (daGal4 > KK control) and dTrmt10A upregulation (daGal4 > UAS-dTrmt10A). Red box highlights loss of 5’UTR m6A with dTrmt10A upregulation. (C) RT-qPCR of Hsp70 RNA levels in basal conditions from control (daGal4 > KK control) and dTrmt10A upregulation (daGal4 > UAS-dTrmt10A) brains. t-test, **p < 0.01. (D) Upregulation of dTrmt10A increased protein levels of Hsp70 in basal and heat stress conditions. daGal4 > KK control vs daGal4 > UAS-dTrmt10A, elavGal4 > KK control vs elavGal4 > UAS-dTrmt10A t-test, *p < 0.05. (E) Upregulation of dTrmt10A (daGal4 > Control vs. UAS-dTrmt10A or elavGal4 > Control vs UAS-dTrmt10A) increased animal heat stress survival. t-test, ****p < 0.0001, ***p < 0.001. (F) Hsp70 protein levels from brains. elavGal4;mCherry RNAi > UAS-mcd8GFP vs elavGal4;Mettl3 RNAi > UAS-mcd8-GFP vs elavGal4;mCherry RNAi > UAS-dTrmt10A vs ElavGal4;Mettl3 RNAi > UAS-dTrmt10A, n = 3 biological replicates, *p < 0.05, **p < 0.01 One-way ANOVA. (G) Genome browser tracks of m6A-IP and Input reads for DnaJ-1 in basal conditions, from control and UAS-dTrmt10A. Red box highlights loss of 5’UTR m6A with dTrmt10A upregulation. (H) Upregulation of dTrmt10A increased protein levels of DnaJ-1 in basal and HS conditions. t-test, *p < 0.05, ***p < 0.001. RADAR differential peak analysis in Supplemental Data 2. (I) RNA decay assay from Control, and UAS-dTrmt10A, in the presence of actinomycin D, to determine relative RNA levels of RpL32 and DnaJ-1. Data are presented as mean ± SD of n = 4 biological replicates, 12 brains per replicate. ****p < 0.0001. Student’s two-tailed t-test. ns = not significant. (J) UAS-dTrmt10A-cat increased protein levels of Hsp70 in basal brain conditions. daGal4 > KK control vs daGal4 > UAS-dTrmt10A-cat, t-test, *p < 0.05. (K) daGAL4 > UAS-dTrmt10A-cat mutant increased heat stress resilience of the animals. Animals heat stressed for 1 h at 38.5 °C and allowed to recover for 24 h. One-way ANOVA, **p < 0.01. (L) Hsp70 protein levels in basal brains with knockdown of additional tRNA methyltransferases. daGal4 > mCherry RNAi, daGal4 > CG9386 RNAi, daGal4 > Mt2 RNAi, daGal4 > Trm7-32 RNAi, One-way ANOVA, ns = not significant.
Similar to Mettl3 RNAi brains, dTrmt10A upregulation significantly reduced 5’UTR m6A on DnaJ-1(Hsp40 chaperone), and UAS-dTrmt10A brains had increased protein levels of DnaJ-1 (Fig. 3G,H). RNA decay analysis indicated upregulation of dTrmt10A led to higher stability of DnaJ-1 RNA while there was no significant difference observed in the RNA levels of the unmodified RpL32 transcript (Fig. 3I). Overall, dTrmt10A upregulation showed similar effects on heat shock chaperone levels and heat shock resilience as observed with Mettl3 RNAi46. Elevated levels of stress chaperones at baseline in the brain potentially precondition the brain to acute heat stress. Taken together, these data highlight that upregulation of dTrmt10A reduces m6A levels and confers heat stress resilience.
Because dTrmt10A is a tRNA methyltransferase, we performed additional experiments to confirm that its role to modulate Hsp70 and the stress response is due to an impact on m6A, and not its role as a tRNA methyltransferase. We generated animals that expressed dTrmt10A with a known mutation in the conserved m1G catalytic domain of the methyltransferase activity that abolishes tRNA methyltransferase activity in vitro14 (G206R; see Fig. 1A). We assessed the impact of this mutation using sensitive readouts of dTrmt10A function: the levels of Hsp70 and animal stress sensitivity. Baseline levels of Hsp70 were increased in the brains of the animals expressing the catalytic mutant (UAS-dTrmt10a-cat), and these animals had increased stress resilience comparable to wild type (Fig. 3J,K). These data indicate that the phenotypic effects of dTrmt10A on Hsp70 and stress resilience are not dependent on the m1G catalytic activity. We also examined three additional tRNA methyltransferases for effects on Hsp70 levels. RNAi of these additional tRNA methyltransferases (CG9386 RNAi, Mt2 RNAi, Trm7-32 RNAi) had no effect on basal Hsp70 levels in the brain (Fig. 3L). Together these data indicate that the effects of dTrmt10A on Hsp70 levels and stress resilience are independent of its catalytic activity as a tRNA methyltransferase, and are not a general property of compromised tRNA methyltransferase activity.
Upregulation of dTrmt10A mimics loss of methyltransferase Mettl3, and globally decreases m6A on neurogenesis and signaling transcripts.
A total of 675 transcripts showed differential m6A methylation upon dTrmt10A upregulation, and only a few of these transcripts were HS chaperones (Fig. 4A). To investigate dTrmt10A further, we compared the m6A sites from dTrmt10A upregulation m6A-IP to the defined Mettl3-dependent methyltransferase m6A sites and transcripts46. 62% of the transcripts from genes with Mettl3-dependent m6A were also regulated by dTrmt10A (Fig. 4A). We focused on these transcripts, as m6A is Mettl3-dependent. Closer analysis of these methylated transcripts revealed 41% of dTrmt10A-dependent m6A sites were in the 5'UTR, while 48% were in the 3'UTR (Fig. 4B), with a small number (81 genes) showing both 5'UTR and 3'UTR-dependent m6A changes (Fig. 4C). Modulation of m6A on the 3’UTR of transcripts was surprising due to the enrichment of m6A on the 5’UTR in Drosophila3,46,47,48. This finding suggests that dTrmt10A may have a specialized role to regulate 3'UTR m6A. When we examined all Mettl3-dependent m6A transcripts with peaks in either the 5’UTR (Fig. 4D) or 3’UTR (Fig. 4E) regions; there was a similar shift towards decreased m6A-IP/Input enrichment upon dTrmt10A upregulation for both regions of transcripts. Reactome and KEGG49,50 pathway analyses showed that these transcripts are enriched for dynamic signaling pathways and axon guidance pathways (Fig. 4F and Supplementary Data 3). Similar GO terms were enriched regardless of 5’UTR or 3’UTR modification site.
dTrmt10A upregulation decreases m6A on neurogenesis and signaling pathway transcripts. (A) Overlap of dTrmt10A-dependent m6A from the m6A-IP sequencing experiment, and Mettl3-dependent m6A transcripts (from46). We focused on the 680 transcripts that are overlapping as dTrmt10A-dependent targets. (B) Percent location of dTrmt10A-dependent m6A at baseline indicates an enrichment for 3’UTR m6A regulation. (C) Overlap of dTrmt10A m6A targets with 5’UTR peaks and those with 3’UTR peaks; some transcripts have m6A in both locations. List of genes given in Supplemental Data 2. (D–E) Scatterplot of normalized read coverage of m6A-IP/input for 5’UTR (left) or 3’UTR (right) of m6A transcripts in control versus UAS-dTrmt10A conditions. dTrmt10A upregulation shows a shift towards decreased m6A levels. (F) Pathway analysis and Kegg term49,50 analysis of all dTrmt10A-dependent m6A transcripts shows enrichment for signaling and neuronal pathways. (G) IGV genome browser tracks of m6A-IP enrichment versus Input reads for Nrg, Myc, and fl(2)d in basal conditions, from control and UAS-dTrmt10A. Red box highlights loss of 5’UTR or 3’UTR m6A with dTrmt10A upregulation. (H) Nrg, Myc, fl(2)d protein levels from control or UAS-dTrmt10A brains dissected in basal conditions. Quantification of 3-biological replicate immunoblots, 15 brains per replicate, showing increased expression of protein in (daGal4 > UAS-dTrmt10A) fly brain. Data are presented as mean ± SD, t-test *p < 0.05.
We examined select targets with available antibodies (Nrg, Myc, and fl(2)d) to determine whether dTrmt10A-dependent m6A modification of transcripts impacted protein levels. Upregulation of dTrmt10A showed decreased m6A on these transcripts, and we observed increased protein levels in Drosophila brain tissue (Fig. 4G,H). These findings support previous work in mammalian cells that observe changes in Myc levels due to m6A-TRMT10A interaction27. We extended these data to global protein synthesis using a puromycin assay to assess nascent protein synthesis. These data showed that upregulation of dTrmt10A was associated with increased puromycin incorporation, thus increased global nascent protein translation in the brain (Supplementary Fig. 1D). We observed the opposite response in puromycin levels upon dTrmt10A RNAi (Supplementary Fig. 1D). The catalytic mutant animals still showed increased global protein synthesis in the brain by puromycin assay (Supplementary Fig. 1E), confirming that puromycin changes are not due to m1G activity of dTrmt10A. The additional tRNA methyltransferases, which did not impact Hsp70 protein levels, also did not affect global puromycin incorporation (Supplementary Fig. 1F). Therefore, dTrmt10A modulation of m6A transcripts likely leads to the influence on overall protein levels in the brain.
Many chromatin regulatory RNAs are m6A modified, and m6A methyltransferase complex components are known to bind chromatin and regulate transcription elements and histone modifications51,52. We therefore considered whether dTrmt10A may also be associated with chromatin. dTrmt10A was overexpressed in the Drosophila salivary glands and dTrmt10A protein localization was examined on the large polytene chromosomes by immunostaining to the HA tag. dTrmt10A showed robust localization to the nucleolus as anticipated for its role in tRNA modification; however, dTrmt10A also showed binding to band locations on the polytene chromosomes (Supplementary Fig. 2A). Chromatin immunoprecipitation followed by western immunoblot validated dTrmt10A association with chromatin (Supplementary Fig. 2B) and chromatin binding was not dependent on RNA (Supplementary Fig. 2C). These data indicate that dTrmt10A is positioned for an additional layer to m6A-modified gene regulation on chromatin, similar to Mettl351,53.
With stress, dTrmt10A primarily impacts 3’UTR m6A sites
Based on the heat stress resilience of upregulated dTrmt10A, we looked more closely at the regulation of m6A during heat stress with overexpression of dTrmt10A. We performed acute heat shock (HS) experiments then analyzed m6A-IP from Drosophila head polyA + RNA. These data showed that dTrmt10A upregulation reduced overall m6A levels in both basal and HS conditions (Fig. 5A). Interestingly, dTrmt10A upregulation led to modification of mostly 3'UTR m6A sites during heat stress, with 73% of dTrmt10A-dependent sites located in this region of transcripts upon HS (Fig. 5B).
dTrmt10A upregulation shows more 3’UTR m6A peak changes with heat stress (HS). (A) Normalized read coverage plot of m6A-IP/input on polyA + transcripts in the 5’UTR, CDS, and 3’UTR of m6A modified transcripts. m6A-IP sequencing in basal and HS conditions from control (daGal4 > KK control) versus dTrmt10A upregulation (daGal4 > UAS-dTrmt10A) heads. (B) Location of dTrmt10A-dependent m6A indicates enrichment for 3’UTR m6A regulation upon HS. (C) Plot of significantly differentially expressed genes in daGal4 > KK control vs > UAS-dTrmt10A heads in HS condition. Positive logFC indicates an increase in transcript levels in dTrmt10A upregulation heads. m6A genes (blue), or all other genes (black). (D) Venn diagram of 5’UTR or 3’UTR dTrmt10A m6A marked transcripts with genes that are differentially expressed in control vs UAS-dTrmt10A in HS conditions. (E) IGV genome browser tracks of m6A-IP enrichment versus Input reads for transcripts in basal conditions, from KK control and UAS-dTrmt10A. Bottom four lanes depict IGV tracks from mCherry RNAi control and Mettl3 RNAi in basal conditions. These peaks show dependence on Mettl3 and UAS-dTrmt10A by RADAR analysis. Red box highlights 3’UTR m6A. Values denote data range on IGV browser for either UAS-dTrmt10A or Mettl3.RNAi sequencing experiments.
Upon heat stress and dTrmt10A upregulation, there was a shift towards increased expression of m6A marked transcripts (Fig. 5C, blue vs black). Furthermore, the majority of these differentially expressed transcripts exhibited dTrmt10A-dependent m6A modifications on their 3' UTR (Fig. 5D). Notably, there was no trend in the levels of non-m6A modified transcripts, with equal numbers of transcripts being up and downregulated between control and dTrmt10A upregulation (Fig. 5C). Among the m6A transcripts, we identified critical genes involved in signaling processes and neurogenesis, such as pAbp, SK, para, Sh, bru3 and even the nuclear m6A reader protein Ythdc1 (Fig. 5E and Supplementary Fig. 3A). These findings highlight the essential role of dTrmt10A in m6A modification during stress, particularly in modifying transcripts that are integral to neuronal signaling processes.
Compared to other tissues, many genes in the Drosophila nervous system have substantially extended 3’UTRs and alternative polyadenylation54,55. We found that 25% of Drosophila CNS transcripts with extended 3’UTRs also have m6A modification in their 3’UTR (Supplementary Fig. 3B). Furthermore, this subset of m6A transcripts are enriched for cognition, learning, and memory pathways, indicating this subclass of 3’UTR m6A modified transcripts are alternatively polyadenylated and involved in learning and memory. We also examined the overall length of 3’UTRs of m6A modified transcripts and found that the average length of their 3’UTR was longer compared to all 3’UTR weighted lengths from wildtype Drosophila CNS55 (Supplementary Fig. 3C).
Proteins that interact with dTrmt10A
In mammalian cells, TRMT10A regulates m6A by interacting with the m6A demethylase FTO. Although there are no known homologs in Drosophila to the demethylases FTO or ALKBH5, we considered that a fly demethylase, should it exist24,27,56, may interact with dTrmt10A. To define interacting proteins, we expressed UAS-dTrmt10A-HA and immunoprecipitated dTrmt10A from Drosophila head lysates using the HA tag. Mass spectrometry was used to evaluate enriched protein interactions of dTrmt10A-HA compared to IgG control (Supplementary Data 4). This analysis identified 6 high confidence interacting proteins (Pp1-87B, CG18680, CG9953, hrm, Dhx15), with no apparent homologs to FTO or ALKBH5. When we analyzed all interactions (low, medium, and high), there was enrichment for proteins involved in RNA metabolism and deadenylation, including DCP1, Atx2, twin, and Rga, as well as proteins involved in translation regulation, such as eIF3a (Fig. 6A). These proteins may interact with and regulate downstream m6A-modified RNA processing through interaction with dTrmt10A. We also investigated whether dTrmt10A binds or alters the levels of known m6A regulators such as Mettl3 or m6A reader proteins Ythdc1 or Fmr1, but found no interaction or altered protein levels (Supplementary Fig. 4), indicating that dTrmt10A does not interact with these known m6A binding proteins to mediate its effect on m6A.
dTrmt10A co-IP interactions from head lysates. (A) Affinity purification of HA–tagged dTrmt10A followed by protein mass spectrometry to identify dTrmt10A-associated proteins. GO term analysis of proteins shown to have a positive interaction with dTrmt10A. Genes above 1.3 LogFC selected as positive interaction. −log10(pval) enrichment of genes and number of genes in each category is shown. See Supplementary Data 4 for full protein list and GO terms with p-values. (B) Human FTO CLIP targets (Ontiveros, R Jordan et al. 202027), overlapped with m6A gene targets modified in the 5′UTR or 3′UTR m6A by upregulation of dTrmt10A. Mass spectrometry proteins and GO analysis genes listed in Supplemental Data 4.
Both mammalian and Drosophila m6A-modified transcripts are enriched for similar biological pathways22,30,57, highlighting the importance of m6A in signaling and stress. Although a candidate demethylase is not identified in Drosophila, we used FTO CLIP targets from mammalian cells27 to define the homologous Drosophila genes. We compared these FTO CLIP targets to dTrmt10A-dependent m6A transcripts and found that ~ 23% of dTrmt10A-dependent m6A transcripts overlap with FTO targets (Fig. 6B). This overlap is similar to the extent of overlap of transcript targets between TRMT10A and FTO in mammalian cells (35%27). Notably, 3’UTR m6A transcripts showed a greater overlap with FTO targets (Fig. 6B). These data suggest that the interaction of dTrmt10A toward 3’UTR m6A RNA binding proteins and/or a putative demethylase may fine-tune aspects of RNA processing.
Discussion
This study offers valuable insights into the in vivo role of dTrmt10A on m6A modification within the Drosophila brain, and its impact on stress resilience and gene regulation. Of note, dTrmt10A modulates m6A peaks both on the 5’UTR of transcripts (where most m6A peaks are in Drosophila transcripts), but also on the 3’UTR of Drosophila genes. Upregulation of dTrmt10A caused a global decrease in m6A levels on transcripts and increased the animal’s resilience to heat stress, with increases in overall protein and RNA levels of key players like Hsp70 (see Figs. 3 and 4). These findings highlight that Drosophila transcripts are modulated by m6A in the 3’UTR in addition to 5’UTR, confirming that m6A modification plays a crucial role in dampening the biological response of the brain to acute stress, and that dTrmt10A acts as an additional regulator of m6A modification of transcripts in the brain. Moreover, modulation of dTrmt10A showed the ability to modulate m6A peak levels in transcripts, despite not being a canonical component of the methyltransferase complex (Fig. 7). dTrmt10A with a mutation in the m1G catalytic domain of the tRNA methyltransferase functioned similarly in our assays as the wild-type protein, and additional Drosophila tRNA methyltransferases failed to show these phenotypes, confirming that the function of dTrmt10A as a tRNA methyltransferase is not influencing Hsp70 protein levels and protein translation. There are many known mutations in human TRMT10A that impact brain development13,14,15, and these data now indicate that the function of TRMT10A/dTrmt10A on m6A levels may also play a role in these diseases.
Model of dTrmt10A regulation in the brain. m6A is highly enriched in the Drosophila brain, and marks transcripts enriched in dynamic signaling and neuronal growth pathways. dTrmt10A upregulation reduces m6A levels in the 5’UTR and 3’UTR of transcripts. Myc and Hsp70 are example genes that show increased protein levels with dTrmt10A upregulation. dTrmt10A upregulation leads to increased RNA and protein levels of these transcripts. Conversely, dTrmt10A knockdown shows a dramatic increase in 5’UTR m6A, with significance for Hsp70. dTrmt10A upregulation modulates heat stress chaperones of Drosophila such that animals are more stress resilient.
m6A modification in brain homeostasis
Many biological functions in the brain are regulated by RNA modification, including axon growth, embryonic stem cell fate, learning and memory, and neurogenesis30,58. Furthermore, mutations in RNA modification proteins have been linked to compromised cellular stress responses, tumorigenesis, and severe developmental defects9,10,20,33,38. Our findings contribute to the current knowledge of RNA modifications regulating the neuronal response to cellular stress, and the regulation of m6A overall in the brain. dTrmt10A regulated m6A levels on signaling pathway transcripts, such as MAPK and WNT signaling pathways that are critical for normal neuronal homeostasis. These signaling pathway transcripts were also regulated by Mettl3, the m6A methyltransferase. We validated m6A changes using Mettl3 knockdown and cross compared to other Drosophila studies; future non-antibody detection techniques would be important to apply.
Although m6A methylation primarily occurs in the 5’UTR of transcripts in Drosophila, dTrmt10A upregulation surprisingly skewed towards selective 3’UTR m6A regulation. Thus far, little is understood regarding why m6A is highly enriched in the 5’UTR of Drosophila transcripts vs 3’UTR as in mammals; however, dTrmt10A has uncovered that m6A regulation occurs in the 3’UTR of transcripts in Drosophila. Many critical signaling and neurogenesis transcripts such as Sh, bru3, para, and pAbp contain dTrmt10A-dependent m6A in the 3’UTR. Additionally, these transcripts were upregulated in brains with increased dTrmt10A expression, indicating they become more stabilized by loss of m6A modification. Transcripts of Myc and Nrg with 3’UTR m6A showed increased protein levels with dTrmt10A upregulation. These findings suggest that both 3’UTR and 5’UTR m6A serve functionally to keep transcript and protein levels low in the Drosophila brain. Furthermore, m6A marked transcripts are enriched in neural and dynamic signaling processes, indicating that one mechanism by which the brain regulates precise dynamics of neural signaling pathways may be through marking key transcripts with m6A for tighter control. Dysfunction in m6A is therefore predicted to have a larger impact on those transcripts critical for neural function. Additionally, we find that many 3'UTR m6A transcripts also have extended 3’UTR lengths in the Drosophila nervous system. A detailed analysis of dTrmt10A effect on alternative polyadenylation may be of interest, particularly within the context of the Drosophila nervous system, given the enrichment of the transcripts in learning, memory, and cognition.
We speculated that dTrmt10A may interact with a demethylase, similar to the mammalian enzyme. By mass spectrometry analysis of dTrmt10A IPed from head lysates and defining the interacting proteins, an obvious candidate was not revealed. The absence of a demethylase homologous to FTO in the fly suggests alternative mechanisms may be at play, such as interactions with RNA-binding proteins involved in decay. Additionally, dTrmt10A could impact proteins within the methyltransferase complex, which themselves have a more direct role on m6A modification; for example, dTrmt10A modulated fl(2)d levels. The mass spectrometry analysis showed dTrmt10A associates with key RNA degradation components including DCP1, Twin, and Rga. Thus, through interaction with dTrmt10A, these proteins may confer specificity and degradation impact of dTrmt10A on m6A targets. Furthermore, disease-associated protein Atx2, mutated in spinocerebellar ataxia 2, was found to co-IP with dTrmt10A. Atx2 is involved in RNA metabolism including RNA stability, RNA localization to stress granules and P-bodies, and interacts with other disease-associated RNA binding proteins such as TDP-4359. We also compared dTrmt10A-m6A modified transcripts to human FTO CLIP RNA targets. There was a significant overlap, and the degree of overlap was comparable to that of mammalian cells27. The overlapped targets are enriched for signaling pathways, similar to GO term analysis of all m6A targets46. Considering that Drosophila m6A is predominantly deposited in the 5’UTR, we were surprised to find more m6A transcripts modified in the 3’UTR that overlapped with FTO targets, indicating specificity in potential demethylase/degradation targeting.
Cellular stress and m6A modifications
To reduce cellular burden during stress, global protein synthesis is repressed and non-essential RNAs are decayed or stored in stalled mRNA-protein granules. During this process, vital RNAs are translated for stress survival. In the recovery phase, stress mediated RNAs are in turn decayed and RNAs necessary for normal cell maintenance are once again transcribed and translated36. The process of stress response activation and resolution is a fine-tuned dance of RNA decay and translation, such that prolonged stress or failure to activate stress machinery is detrimental. RNA modifications have emerged as critical regulators of dynamic signaling in normal cell maintenance and also during stress recovery20,31,38. Particularly neurons, which are postmitotic and cannot remove harmful accumulations through cell division, show specialized mechanisms to combat cellular stress.
In mammalian cells m6A modification has been shown to increase upon heat stress in the 5’UTR of Hsp70 to facilitate cap-independent translation26. Other studies in vitro indicate that Hsp70 becomes co-transcriptionally marked by m6A, which leads to its degradation39. Furthermore during stress resolution, the lncRNA Heat, a transcriptional activator of HSF1, becomes heavily methylated by m6A, and Ythdc1 degrades the lncRNA to prevent stress gene activation40. In the Drosophila brain, Hsp70 is m6A modified in the 5’UTR at baseline, and m6A is lost upon acute heat stress46. We hypothesize this limits Hsp70 translation at baseline and that the loss of m6A facilitates accumulation of the transcript for translation during stress. Thus, loss of m6A in the 5’UTR of Hsp70 leads to elevated levels of Hsp70 protein, which may allow for a state of preconditioning of the brain to future stress. With dTrmt10A knockdown, we were surprised to see a dramatic increase in 5’UTR m6A on Hsp70—especially given that no other transcripts showed such a dramatic increase in m6A. These data indicate that stress resilience may be a particularly important target of TRMT10A/dTrmt10A gene mutation.
In summary, using m6A-IP sequencing, we find that dTrmt10A impacts 5’UTR m6A methylation on key stress chaperones, but dTrmt10A also highlights that 3’UTR m6A occurs and is regulated in Drosophila. These studies provide a framework for future insight into how m6A regulation is achieved at specific sites, like the 5’UTR vs 3’UTR. These data reveal that additional m6A regulators like dTrmt10A outside of the core complex components may profoundly modulate region specific methylation and/or demethylation. m6A regulation in the brain likely serves to fine tune signaling pathways for the continued maintenance of axons and dynamic synaptic connections. This comes at the cost of dampening the acute stress response, which compromises the overall ability of the brain to withstand insults. Given that mutations in TRMT10A are associated with developmental brain disease, some mutations that compromise TRMT10A function may also include impacts on m6A and stress resistance.
Materials and methods
Drosophila stocks and maintenance
A full list of Drosophila stocks used in this study are described in Supplementary Data 1. RNAi and UAS lines were generated by the Harvard Transgenic RNAi Project (TRiP) and the Vienna Drosophila Resource Center (VRDC)60,61, and stocks were obtained from the Bloomington Drosophila Stock Center, Indiana, USA. Crosses were performed at 25 °C and grown on standard cornmeal molasses agar. Driver lines used: daGAL4, elavGal4, nSybGal4 as indicated per experiment. For all experiments male flies were used, for consistency in the experiments, and to avoid issues in food consistency due to egg laying of females.
Brain dissections
Brain dissections were conducted as previously described62. Briefly, flies were anesthetized using CO2 and decapitated using forceps. The head was placed posterior side down and the proboscis was then removed using Dumont #5 fine forceps (Fine Science Tools, 11254-20). The brain was then gently popped out through the proboscis cavity, cleaned in PBS, and transferred to an RNAse free microfuge tube and PBS was aspirated. Brains were then ground in Laemmli Buffer (5 μL per brain, 10–20 brains for each sample) for Western immunoblotting, or in Trizol for RNA analysis.
Western immunoblot analysis
Brain or head samples were homogenized in sample buffer of 1 × Laemmli sample Buffer (Bio-rad, 1610737), 50ul b-mercaptoethanol (Sigma, m6250), 1 × protease inhibitor (Roche, 11836170001), and 1 mM PMSF (Sigma, P7626). 5ul of sample buffer is added per brain, 7.5 ul added per head, and 40ul added per whole fly. Samples are boiled at 98 °C for 3 min, and then centrifuged at 1500 rpm for 3 min at room temp. Sample was loaded onto 15 well 1.0 mm 4%–12% Bis-Tris NuPAGE gels (Thermo Fisher, WG1401) with pre-stained protein ladder (Thermo Scientific, 22619). 1 brain, 1 head, or 8% of whole fly tissue is loaded on each lane per experiment. Gel electrophoresis was performed using Xcell Surelock Mini-Cell Electrophoresis System at 140 V, and transferred overnight onto a nitrocellulose membrane 0.45 μM (Bio-rad, 1,620,115), using a Bio-rad mini transblot cell at 90A for 16 h. Membranes were stained in Ponceau S (Sigma, P7170-1L), washed in DI water, and imaged with Amersham Imager 600. Ponceau S was washed off in 3 × 5 min in Tris-buffered saline with 0.1% Tween20 (TBST). Membrane was blocked in 5% non-fat dry milk (LabScientific, M08410) in TBST for 1 h, and incubated with primary antibodies with blocking buffer overnight at 4 °C. Following 3 × 5 min washed in TBST, membranes were incubated with HRP-conjugated secondary antibodies at 1:5,000 for 1 h at room temp in blocking solution. Membranes were washed 3 × 5 min in TBST and the signal was developed using ECL prime (Cytivia, #RPN2232) and detected using an Amersham Imager 600. Primary antibodies used: anti-tubulin (1:5000, DHSB, #AA4.3, Lot.5/31/18-44ug/ml), anti-Hsp70 (1:5000, Sigma, #7FB-SAB5200204-100uG, Lot.141002), anti-Mettl3 (1:5000, Proteintech, #15073-1-AP, Lot.Ag7110), anti-HSP40 (1:5000, Enzo Life Sciences, #ADI-SPA-400-D, Lot.04062141), anti-fl(2)d (1:10, #DSHB-9G2, Lot.10/18/18-42ug/ml), anti-dFmr1 (1:500, #DSHB-5A11), anti-Nrg (1:600, #DSHB-BP104), anti-Myc (1:50, #DSHB-P4C4-B10), anti-puromycin (1:1000, Kerafast, #EQ0001, Lot.200517). Rabbit anti-Ythdc1 (1:5000) was created by Vivitide against 18 residues of Ythdc1 (157–173 “CRTKIPSNANDSAGHKSD”)46. Secondary Antibodies used: Goat anti-mouse (1:5000, Jackson lmmunoResearch, #115-035-146, Lot.153978), Goat anti-Rabbit (1:5000, Jackson lmmunoResearch, #111-035-144, Lot.138306), Goat anti-rat (1:5000, Thermo Fisher Scientific, #A10549, Lot.2273679).
RNA extraction
Tissue was homogenized in 200ul of Trizol (Thermo Scientific, #15596026) in RNase free 1.5 ml microfuge tubes (Thermo Scientific, #AM12400). 800ul of Trizol (Thermo Scientific, #15596026) was added to the tube and 200ul of chloroform (Fisher Scientific, #AC423555000) and was vigorously shaken for 20 s at room temp. Samples were left for 5 min at RT to form upper aqueous phase, and centrifuges at 4 °C for 15 min at 12,000×g. The Upper aqueous phase was transferred to a fresh RNase free tube. RNA samples were then processed using the Zymo RNA clean & concentrator -5 kit (Zymo, #R1013), using their RNA clean-up from aqueous phase after Trizol /chloroform extraction protocol plus on column DNase I treatment. RNA amount was measured using a nanodrop and integrity was validated through on an Agilent 2100 Bioanalyzer using an RNA nano chip.
Real time PCR
400 ng RNA was used per cDNA reaction using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Thermo Scientific, #4368814). cDNA was then used for qPCR reactions set up with SYBR Green Fast Reagents, using a 384-well plates on the Applied Biosystems ViiA7 machine. Primers used are in Supplementary Data 1. Mean fold change was determined using the ∆∆Ct method. Each experiment used technical triplicates as well as three biological replicates, Graphpad prism 8/9 software was used for statistics.
Stress sensitivity assay
Fly crosses were carried out at 25 °C. Adult flies were collected and aged to 6d post eclosion. Flies were anesthetized and transferred to clear plastic 13 ml vials, and cotton was placed at the 4 ml mark on the vials to concentrate the flies near the bottom. Each vial contained 20 flies. Flies were allowed to recover for 30 min, and then transferred to a water bath for mild non-lethal heat stress (30 min at 38.5 °C) or a longer heat shock (1–1.5 h at 38.5 °C) for a severe stressor to measure stress sensitivity. The flies were then transferred to normal food and allowed to recover overnight at 25 °C. After recovery, the percent of flies alive versus dead was recorded per vial.
LC–MS/MS analysis of m6A levels
PolyA + RNA was extracted from brains, heads, and whole fly RNA using the NEBNext Poly(A) mRNA Magnetic Isolation Module (NEB, #E7490L). LC–MS/MS was conducted as previously described27. All quantifications were performed by using the standard curve obtained from pure nucleoside standards running with the same group of samples. Then, the percentage ratio of m6A to A or m1G to G was used to compare the different modification levels.
m6A-IP sequencing
Total RNA was extracted from 200 Drosophila heads per replicate using Trizol/ chloroform extraction. PolyA + mRNA was obtained using NEBNext Poly(A) mRNA Magnetic Isolation Module. PolyA + RNA was fragmented using the NEBNext Magnesium Fragmentation Module (NEB, #E6150S) for 4 min at 95 °C for a 250 ng sample of polyA + RNA, and RNA was repurified using the Zymo RNA clean & concentrator -5 kit (Zymo, #R1013). 10% of the fragmented polyA + RNA was saved as an input control for sequencing. M6A -immunoprecipitation was done using the EpiMark N6-Methyladenosine Enrichment kit protocol with some minor alterations described. 30ul of protein G magnetic beads (NEB, #S1430) were washed and resuspended in IP buffer (150 mM NaCl, 10 mM Tris–HCL, 0.1% NP-40). 4ul of Synaptic Systems antibody (Synaptic Systems, #202003, Lot.2-119) was conjugated to protein G-magnetic beads (NEB, #E1611A, Lot.10015190) for 2 h at 4 °C. Beads/ antibody were washed twice in IP buffer. ~ 1 μg polyA + RNA was incubated with beads/antibody in IP buffer supplemented with 0.1% SUPERase-In RNase Inhibitor (Thermo Scientific; #AM2696) for 2 h at 4 °C. After incubation, RNA/beads/antibody are washed twice in IP buffer, twice in low salt IP buffer (50 mM NaCl, 10 mM Tris–HCL, 0.1% NP-40), and twice in high salt IP buffer (500 mM NaCl, 10 mM Tris–HCL, 0.1% NP-40). RNA is eluted from beads with 25 μl of RLT buffer twice and elution was pooled and concentrated using Zymo RNA clean and concentrator -5 kit (Zymo, #R1013). Libraries were made using SMARTer Stranded Total RNA-Seq Kit V2 without rRNA depletion (Takarabio, #634411) for IPed and input RNA, and sequenced using illumina HiSeq X series with 40M paired end reads (2 × 150 bp). Library preparation and sequencing was done by Admera Health. Three biological replicates per genotype and condition were done with NEB m6A antibody, and two biological replicates were done with Synaptic Systems m6A antibody.
m6A enrichment analysis
Regions of m6A enrichment were found for each condition using MetPeak (v.1.1)63 with default parameters, using the input and m6A pulldown bam files as input, and with the FlyBase FB2019_05 annotation provided. Peak locations (5’ UTR, CDS, or 3′ UTR) were defined from the regions indicated by MetPeak as having significant m6A enrichment. If a peak was not contained in one region (i.e., if the peak is partly in the CDS and partly in the 3’ UTR), it was assigned to the region where more of the peak resided.
Differential m6A peak analysis
Regions of differential methylation between two conditions (frequently called “dTrmt10A-dependent” or “m6A genes”) were found using RADAR (v.0.2.4)64 with input and m6A pulldown bam files as input, as well as the FlyBase FB2019_05 annotation. All replicates were used for differential peak calling. The minimum cutoff for bin filtering was 15, the cutoff was set as 0.05, and the Beta_cutoff was set as 0.5. Any region with an adjusted p-value < 0.05 was retained, and regions with a fold-change < − 1 from the control (KK Control) to the upregulation (UAS-dTrmt10A) at basal or heat shock (30 min) conditions were kept as dTrmt10A-dependent peaks. All other genes expressed in the brain that did not have dTrmt10A-dependent m6A were considered as “all other genes.” Full RADAR differential peaks with no cut-off are listed in Supplementary Data 2.
m6A metaplots and genome browser visualization
Heatmaps and metagene plots showing the location of m6A enrichment on a specific set of genes were constructed with using pheatmaps (v.1.0.12) and meRIPtools (v.0.2.1). Specifically, the exons of all transcripts in each gene were collapsed using the GenomicRanges (v.1.44.0) function reduce65. Genes with a 5′ UTR or 3′ UTR shorter than 30 base pairs, a CDS shorter than 100 bp, or lacking a 5′/3′ UTR (i.e., lncRNAs) were not considered in this analysis. For each gene, the 5’ UTR and 3′ UTR were tiled in 30 evenly spaced bins, and the CDS was tiled in 100 evenly spaced bins. The number of input and m6A reads overlapping each bin was calculated and this number was divided by the bin width and library size and a normalization factor of one million to produce a normalized reads per million in each bin. For genome browser snapshots, separated input and m6A-IP tracks vere visualized. Tracks were made by first converting bam files to bigWig files using deepTools (v.3.5.1)66 bamCoverage using CPM normalization, then deepTools bigwigCompare with operation log2.
GO and pathway analysis
GO analysis for genes with dTrmt10A-dependent m6A was conducted using FlyMine (v.53)67. The test correction was set to Holm-Bonferroni with a max p-value of 0.05. KEGG49,50 pathway analysis was done using the “enrichKEGG” function from ClusterProfiler (v.4.0.5) package in R68. A list of all genes with detectable expression was used as background for both GO and pathway analysis.
RNA-seq analysis
Raw paired-end fastqs were processed with TrimGalore (v.0.6.6) (https://github.com/FelixKrueger/TrimGalore) with default settings to remove Illumina adapters and mapped using STAR 2.7.3a69 to the Drosophila melanogaster genome annotation dm6. Unmapped and improperly paired reads were filtered out of aligned bam files. Reads per gene in the FlyBase release 2019_05 were computed using an R script using GenomicRanges (v.1.44.0)70 function summarizeOverlaps that counts the number of reads overlapping with the exons of each gene in the default “union” mode. Differential expression analysis was performed using DESeq2 (v.1.32.0)71, with count files produced by summarizeOverlaps as input. PCA plots were made using the plotPCA function in DESeq2, with variance stabilized counts as the input. MA plots were constructed from the adjusted p-values and baseMean values output from DESeq2, and volcano plots were constructed from adjusted p-values and fold changes reported by DESeq2. Normalized counts produced by DESeq2 were used to show expression levels. Differentially expressed genes were considered to be any gene with a p-adjusted values of < 0.05.
Actinomycin assay
Brains from KK Control, UAS-dTrmt10A, and Trmt10A RNAi adults were dissected and incubated in 250ul of Schneider's Drosophila Medium that was pre-warmed to 25 °C in RNase free 1.5 ml microfuge tubes. 12–15 brains were dissected for each timepoint. 169.5ug/ml actinomycin D (Sigma-Aldrich, A1410) was added to each sample. Samples were incubated at 25 °C shaking at 300 rpm for 4 h, and mixed by pipetting every 1 h. After the incubation time samples were collected, spun at room temp, washed in cold RNAse free PBS, and processed in Trizol. Brain total RNA was collected using trizol/chloroform and Zymo RNA clean and concentrator kit-5 (R1015). RNA was then used for RT-qPCR. 400 ng of RNA is used per cDNA reaction. cDNA is also quantified by Qubit ssDNA Assay (Invitrogen, Q10212) before use in RT-qPCR experiments.
Puromycin assay
Food was made by mixing 600 µM puromycin (Sigma-Aldrich, P8833-100MG) with 2% agar + 5% sucrose. Adult flies were placed onto puromycin food for 24 h. Brains were dissected from flies after 24 h of feeding and samples were processed in for western blot analysis. Protein loading was determined by Ponceau S stain of membrane.
Quantification and statistical analysis
Statistical tests used were performed on GraphPad Prism (v.9), and are indicated in the figure legend. p-values of < 0.05 were considered significant. Unpaired two-tailed t-tests were used when comparing 2 groups; One-way ANOVA was used when comparing multiple groups followed by Tukey’s post-test when each group was compared against every other group, Sidak’s post-test when pre-defined groups were compared to each other, or Dunnett’s test when comparing to a defined control sample. Two-way ANOVA was used when there were 2 factors in the analysis. One-sided Hypergeometric test was used to compare Venn diagram overlaps.
Salivary gland immunohistochemistry
Third instar larva were dissected in PBST (0.05% Tween 20) and salivary glands were fixed in 2% paraformaldehyde (Electron Microscopy Sciences, #15713) in 45% acetic acid (Alfa Aesar, #36289) for 1 min at room temperature. Fixed salivary glands were loaded in 45% acetic acid on the slide glass and covered with siliconizing reagent (Sigmacote, Sigma-Aldrich, #SL2) coated coverslips to squash polytene chromosomes. Slides were snap frozen with liquid nitrogen and coverslips removed. Samples were blocked with 3% BSA (Sigma, #A7906) solution in PBST for 1 h at room temperature. Primary antibody against HA (Santa cruz, sc-805) and goat anti-rabbit IgG Alexa Fluor488 (Invitrogen, #A11008) were diluted with blocking solution in 1:50 and 1:200 ratio, respectively. VECTASHIELD mounting medium with DAPI (Vector Laboratories, #H-1500) was added at the last step to stain nucleic acids and mount samples. Imaging was conducted on a Leica STELLARIS 5 confocal microscope.
ChIP-WB and RNase treatment
Fly brains (30 brains/sample) were dissected in cold PBS buffer and fixed in 1% formaldehyde (Fisher Scientific, #BP531) for 10 min at room temperature, and washed 3 times with PBS buffer. Fixed brain samples were homogenized in lysis buffer 1(50 mM HEPES pH7.5, 140 mM NaCl, 1 mM EDTA, 10% Glycerol, 0.5% NP-40, 0.25% Triton X-100), and treated with lysis buffer 2 (10 mM Tris pH8, 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA), then chromatin samples were collected after sonication in lysis buffer 3 (10 mM Tris pH 8, 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% Sodium Deoxycholate, 0.5% N-lauroylsarcosine). 10% of chromatin sample was used for input samples. Anti-HA (Santa Cruz, #sc-7392X, 2ug) and normal mouse IgG (Santa cruz, #sc-2025, 2ug) antibodies were added to Dynabeads Protein A (Invitrogen, #10002D) and incubated for 6 h at 4 °C. Then applied beads to chromatin samples for overnight incubation at 4 °C. Beads were washed and then denatured with LDS sample buffer (Invitrogen, #NP0007) at 95 °C for 5 min. Beads and supernatant were separated on a magnet stands, and the supernatant was used to perform western blot. For RNase treatment, RNase A/T1 mix (Thermo Scientific, #EN0551) was added to lysis buffer 2, and incubated with brain pellets for 2 h at 4 °C.
Immunoprecipitation with dTrmt10A
To immunoprecipitate dTrmt10A from adult fly heads the Pierce Co-Immunoprecipitation kit (Thermo Scientific, #26149) was utilized according to manufacturer’s instructions. 15ug either anti-HA antibody (mouse, Santa Cruz, #sc-7392X) or mouse IgG (mouse, Santa Cruz, #sc-2025) was immobilized to agarose resin according to kit instructions. Heads of adult male flies expressing UAS-dTrmt10A-FLAG-HA with daGal4 were isolated and homogenized for 1 min on ice in 200 uL of IP Lysis/wash buffer (Thermo Scientific) that was supplemented with cOmplete Mini EDTA-free protease inhibitor (Roche, #11836170001). 20 uL of sample was removed for input. Remaining sample lysate was pre-cleared with agarose resin (1 h at 4 °C with gentle rocking). Precleared lysate was incubated with antibody-coupled resin overnight (~ 18 h) at 4 °C with gentle rocking. Agarose resin was then washed three times with IP Lysis/wash buffer supplemented with protease inhibitor (10 min each with rotation at 1000×g, 4 °C). IP samples were eluted in either 50 uL for immunoblotting or 30 uL for mass spec analysis. Samples for immunoblotting were prepared in 4 × LDS Sample Buffer (Invitrogen, #NP0007), heat denatured at 90 °C for 6 min and electrophoresed on a 4–12% Bis–Tris gel and transferred to nitrocellulose. dTrmt10A signal was detected using anti-HA-peroxidase antibody (1:500 rat, Roche, #12013819001).
Mass spectrometry analysis of dTrmt10A protein
dTrmt10A was immunoprecipitated from adult fly heads (80 per replicate, n = 3 biological replicates per antibody) as described above using anti-HA antibody or mouse IgG as control. Samples were run on NuPAGE™ 10%, Bis–Tris gel for 0.5 cm, and stained using Imperial™ Protein Stain (ThermoFisher, #24615). Mass spectrometry analysis was done by Wistar Proteomics and Metabolomics Facility. The entire stained gel regions were excised, reduced with TCEP, alkylated with iodoacetamide, and digested with trypsin, and tryptic digests were analyzed using a standard 1.5-h LC gradient on the Thermo Q Exactive Plus mass spectrometer. MS data were searched with full tryptic specificity against the UniProt Drosophila melanogaster proteome database (7/21/2022) plus the sequence provided (dTrmt10A-HA), and a common contaminant database using MaxQuant 1.6.3.3. Fold change was calculated based on intensity of HA signal to IgG signal, and intensity is the sum of the peptide MS peak intensities for the protein. To calculate the fold change, protein intensity values were processed by: Log2 transformation of the intensity values and replacing missing values (proteins not identified) with the minimum value of the dataset, for comparison between two sample groups, Log2 ratio, Fold change and Student's t-test p-value were calculated using the Log2 imputed intensity, the more stringent q-value (t-test p-value adjusted to account for multiple testing using Benjamini–Hochberg FDR) was also calculated. "High" confident identification of proteins with significant change ("Confident" column) refers to proteins satisfying the 4 criteria: minimum absolute fold change of 2, and q-value < 0.1, and identified by a minimum of 2 razor + unique peptides in HA-IP group, and detected in at least 2 of the HA-IP replicates. "Medium" confident protein identifications refer to proteins satisfying the 3 criteria: Identified by a minimum of 2 razor + unique peptides in HA-IP group, and detected in at least 2 of the HA-IP replicates, and not detected in any of the IgG-IP replicates.
Data availability
The Raw sequencing data generated in this study have been deposited in the Gene Expression Omnibus GSE229430. Any additional inquiries can be directed to the corresponding author.
References
Zhao, B. S., Roundtree, I. A. & He, C. Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 18, 31–42 (2017).
Yang, Y., Hsu, P. J., Chen, Y.-S. & Yang, Y.-G. Dynamic transcriptomic m6A decoration: Writers, erasers, readers and functions in RNA metabolism. Cell Res. 28, 616–624 (2018).
Kan, L. et al. A neural m 6 A/Ythdf pathway is required for learning and memory in Drosophila. Nat. Commun. 12, 1458 (2021).
Widagdo, J., Wong, J.J.-L. & Anggono, V. The m6A-epitranscriptome in brain plasticity, learning and memory. Semin. Cell Dev. Biol. 125, 110–121 (2022).
Koranda, J. L. et al. Mettl14 is essential for epitranscriptomic regulation of striatal function and learning. Neuron 99, 283-292.e5 (2018).
Shi, H. et al. m 6 A facilitates hippocampus-dependent learning and memory through YTHDF1. Nature 563, 249–253 (2018).
Zhao, B. S. et al. m6A-dependent maternal mRNA clearance facilitates zebrafish maternal-to-zygotic transition. Nature 542, 475–478 (2017).
Shi, H., Wei, J. & He, C. Where, when and how: Context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell 74, 640–650 (2019).
Cui, Q. et al. m6A RNA methylation regulates the self-renewal and tumorigenesis of glioblastoma stem cells. Cell Rep. 18, 2622–2634 (2017).
Angelova, M. T. et al. The emerging field of epitranscriptomics in neurodevelopmental and neuronal disorders. Front. Bioeng. Biotechnol. 6, 46 (2018).
Nau, F. The methylation of tRNA. Biochimie 58, 629–645 (1976).
Pan, T. Modifications and functional genomics of human transfer RNA. Cell Res. 28, 395–404 (2018).
Cosentino, C. et al. Pancreatic β-cell tRNA hypomethylation and fragmentation link TRMT10A deficiency with diabetes. Nucl. Acids Res. 46, 10302–10318 (2018).
Gillis, D. et al. TRMT10A dysfunction is associated with abnormalities in glucose homeostasis, short stature and microcephaly. J. Med. Genet. 51, 581–586 (2014).
Igoillo-Esteve, M. et al. tRNA methyltransferase homolog gene TRMT10A mutation in young onset diabetes and primary microcephaly in humans. PLoS Genet. 9, e1003888 (2013).
Narayanan, M. et al. Case Report: Compound heterozygous nonsense mutations in TRMT10A are associated with microcephaly, delayed development, and periventricular white matter hyperintensities. F1000Res 4, 912 (2015).
Vilardo, E. et al. Functional characterization of the human tRNA methyltransferases TRMT10A and TRMT10B. Nucl. Acids Res. 48, 6157–6169 (2020).
Hong, J., Xu, K. & Lee, J. H. Biological roles of the RNA m6A modification and its implications in cancer. Exp. Mol. Med. 54, 1822–1832 (2022).
Lence, T., Soller, M. & Roignant, J.-Y. A fly view on the roles and mechanisms of the m6A mRNA modification and its players. RNA Biol. 14, 1232–1240 (2017).
Engel, M. et al. The role of m6A/m-RNA methylation in stress response regulation. Neuron 99, 389-403.e9 (2018).
Anders, M. et al. Dynamic m6A methylation facilitates mRNA triaging to stress granules. Life Sci. Alliance https://doi.org/10.26508/lsa.201800113 (2018).
Weng, Y.-L. et al. Epitranscriptomic m6A regulation of axon regeneration in the adult mammalian nervous system. Neuron 97, 313-325.e6 (2018).
Haussmann, I. U. et al. m 6 A potentiates Sxl alternative pre-mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304 (2016).
Bartosovic, M. et al. N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3′-end processing. Nucl. Acids Res. 45, 11356–11370 (2017).
Shi, H. et al. YTHDF3 facilitates translation and decay of N 6-methyladenosine-modified RNA. Cell Res. 27, 315–328 (2017).
Meyer, K. D. et al. 5′ UTR m6A promotes cap-independent translation. Cell 163, 999–1010 (2015).
Ontiveros, R. J. et al. Coordination of mRNA and tRNA methylations by TRMT10A. PNAS 117, 7782–7791 (2020).
Meyer, K. D. et al. Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell 149, 1635–1646 (2012).
Merkurjev, D. et al. Synaptic N6-methyladenosine (m6A) epitranscriptome reveals functional partitioning of localized transcripts. Nat. Neurosci. 21, 1004–1014 (2018).
Yoon, K.-J. et al. Temporal control of mammalian cortical neurogenesis by m6A methylation. Cell 171, 877-889.e17 (2017).
Edens, B. M. et al. FMRP modulates neural differentiation through m6A-dependent mRNA nuclear export. Cell Rep. 28, 845-854.e5 (2019).
Du, H. et al. YTHDF2 destabilizes m 6 A-containing RNA through direct recruitment of the CCR4–NOT deadenylase complex. Nat. Commun. 7, 12626 (2016).
Li, M. et al. Ythdf2-mediated m6A mRNA clearance modulates neural development in mice. Genome Biol. 19, 69 (2018).
Haigis, M. C. & Yankner, B. A. The aging stress response. Mol. Cell 40, 333–344 (2010).
Kagias, K., Nehammer, C. & Pocock, R. Neuronal responses to physiological stress. Front. Genet. 3, 222 (2012).
Vihervaara, A., Duarte, F. M. & Lis, J. T. Molecular mechanisms driving transcriptional stress responses. Nat. Rev. Genet. 19, 385–397 (2018).
Zaccara, S. & Jaffrey, S. R. A unified model for the function of YTHDF proteins in regulating m6A-modified mRNA. Cell 181, 1582-1595.e18 (2020).
Xiang, Y. et al. RNA m 6 A methylation regulates the ultraviolet-induced DNA damage response. Nature 543, 573–576 (2017).
Knuckles, P. et al. RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat. Struct. Mol. Biol. 24, 561–569 (2017).
Ji, Q., Zong, X., Mao, Y. & Qian, S.-B. A heat shock–responsive lncRNA Heat acts as a HSF1-directed transcriptional brake via m6A modification. PNAS 118, e2102175118 (2021).
Zhou, J. et al. Dynamic m 6 A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594 (2015).
Bonini, N. M. Chaperoning brain degeneration. Proc. Natl. Acad. Sci. U.S.A. 99(Suppl 4), 16407–16411 (2002).
Kan, L. et al. The m6A pathway facilitates sex determination in Drosophila. Nat. Commun. 8, 15737 (2017).
Lence, T. et al. m6A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247 (2016).
Worpenberg, L. et al. Ythdf is a N6-methyladenosine reader that modulates Fmr1 target mRNA selection and restricts axonal growth in Drosophila. EMBO J. 40, e104975 (2021).
Perlegos, A. E., Shields, E. J., Shen, H., Liu, K. F. & Bonini, N. M. Mettl3-dependent m6A modification attenuates the brain stress response in Drosophila. Nat. Commun. 13, 5387 (2022).
Wang, Y. et al. Role of Hakai in m 6 A modification pathway in Drosophila. Nat. Commun. 12, 2159 (2021).
Bawankar, P. et al. Hakai is required for stabilization of core components of the m6A mRNA methylation machinery. Nat. Commun. 12, 3778 (2021).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucl. Acids Res. 28, 27–30 (2000).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucl. Acids Res. 51, D587–D592 (2023).
Akhtar, J. et al. m6A RNA methylation regulates promoter—Proximal pausing of RNA polymerase II. Mol. Cell 81, 3356-3367.e6 (2021).
Liu, J. et al. N6-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science 367, 580–586 (2020).
Xu, W. et al. METTL3 regulates heterochromatin in mouse embryonic stem cells. Nature 591, 317–321 (2021).
Hilgers, V., Lemke, S. B. & Levine, M. ELAV mediates 3′ UTR extension in the Drosophila nervous system. Genes Dev. 26, 2259–2264 (2012).
Wei, L. et al. Overlapping activities of ELAV/Hu family RNA binding proteins specify the extended neuronal 3′ UTR landscape in Drosophila. Mol. Cell 80, 140-155.e6 (2020).
Fu, Y. et al. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat. Commun. 4, 1798 (2013).
Zheng, H., Zhao, Y., Zhou, H., Tang, Y. & Xie, Z. The comprehensive analysis of m6A-associated Anoikis genes in low-grade gliomas. Brain Sci. 13, 1311 (2023).
Batista, P. J. et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell 15, 707–719 (2014).
Ostrowski, L. A., Hall, A. C. & Mekhail, K. Ataxin-2: From RNA control to human health and disease. Genes (Basel) 8, 157 (2017).
Perkins, L. A. et al. The transgenic RNAi project at harvard medical school: Resources and validation. Genetics 201, 843–852 (2015).
Dietzl, G. et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448, 151–156 (2007).
Kennerdell, J. R., Liu, N. & Bonini, N. M. MiR-34 inhibits polycomb repressive complex 2 to modulate chaperone expression and promote healthy brain aging. Nat. Commun. 9, 4188 (2018).
Cui, X., Meng, J., Zhang, S., Chen, Y. & Huang, Y. A novel algorithm for calling mRNA m6A peaks by modeling biological variances in MeRIP-seq data. Bioinformatics 32, i378–i385 (2016).
Zhang, Z. et al. RADAR: Differential analysis of MeRIP-seq data with a random effect model. Genome Biol. 20, 294 (2019).
Zhang, Z. et al. Genetic analyses support the contribution of mRNA N 6-methyladenosine (m 6 A) modification to human disease heritability. Nat. Genetics 52, 939–949 (2020).
Ramírez, F. et al. deepTools2: A next generation web server for deep-sequencing data analysis. Nucl. Acids Res. 44, W160–W165 (2016).
Smith, R. N. et al. InterMine: A flexible data warehouse system for the integration and analysis of heterogeneous biological data. Bioinformatics 28, 3163–3165 (2012).
Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Dobin, A. et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Lawrence, M. et al. Software for computing and annotating genomic ranges. PLoS Comput. Biol. 9, e1003118 (2013).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Acknowledgements
We thank the Drosophila Genomics Resource Center (NIH grant 2P40OD010949), the Bloomington Drosophila Stock Center (NIH P40OD018537) and the Transgenic RNAi Project (TRiP) at Harvard Medical School (NIH/NIGMS R01-GM084947). We thank the Proteomics and Metabolomics Facility at The Wistar Institute. This work was supported by T32-GM007229 and F31-AG063470 (to AEP), R35-GM133721 (to KFL) and R35-NS097275 (to NMB).
Author information
Authors and Affiliations
Contributions
A.E.P. conceived, designed, and performed experiments, statistical analysis, bioinformatic analysis, and analyzed data. X.Q. performed chromatin experiments. K.M.D performed immunoprecipitations and assisted in experiments. E.J.S. designed bioinformatic pipeline. H.S. and H.E. performed mass spec experiments. K.F.L. supervised research and analyzed data. N.M.B. conceived, designed experiments, analyzed data, and supervised the research. K.F.L and H.S. gave input and edited the manuscript. A.E.P and N.M.B. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Perlegos, A.E., Quan, X., Donnelly, K.M. et al. dTrmt10A impacts Hsp70 chaperone m6A levels and the stress response in the Drosophila brain. Sci Rep 13, 22999 (2023). https://doi.org/10.1038/s41598-023-50272-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-023-50272-4
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.