Abstract
Pneumonia caused by the intracellular bacterium Rhodococcus equi is an important cause of disease and death in immunocompromised hosts, especially foals. Antibiotics are the standard of care for treating R. equi pneumonia in foals, and adjunctive therapies are needed. We tested whether nebulization with TLR agonists (PUL-042) in foals would improve innate immunity and reduce the severity and duration of pneumonia following R. equi infection. Neonatal foals (n = 48) were nebulized with either PUL-042 or vehicle, and their lung cells infected ex vivo. PUL-042 increased inflammatory cytokines in BAL fluid and alveolar macrophages after ex vivo infection with R. equi. Then, the in vivo effects of PUL-042 on clinical signs of pneumonia were examined in 22 additional foals after intrabronchial challenge with R. equi. Foals infected and nebulized with PUL-042 or vehicle alone had a shorter duration of clinical signs of pneumonia and smaller pulmonary lesions when compared to non-nebulized foals. Our results demonstrate that host-directed therapy can enhance neonatal immune responses against respiratory pathogens and reduce the duration and severity of R. equi pneumonia.
Similar content being viewed by others
Introduction
Rhodococcus equi is a facultative, intracellular bacterium that preferentially replicates within macrophages1 and causes a pyogranulomatous pneumonia in foals and immunocompromised people that resembles tuberculosis. Infection of foals with R. equi occurs during the first weeks after birth2, when foals are more susceptible to infection with this pathogen3, probably because of the naivety/immaturity of both innate and adaptive immune responses4,5,6,7,8,9. Adaptive immune responses require more time to develop such that neonates of all species have a window during which they are not able to be protected by vaccines and consequently depend heavily on innate immune responses10. Even if foals could generate an effective adaptive immune response to vaccination, no vaccine against R. equi pneumonia is commercially available despite decades of research. There is evidence that maternal vaccination against R. equi protects foals against clinical pneumonia11; that approach, however, has some limitations such as it is dependent on the dam’s immune status and adequate immune response to vaccination, ingestion of sufficient colostrum in the first 24–36 h after birth (there is no transfer of immunoglobulins through the placenta in the equine species), etc. Other preventive methods such as transfusion of R. equi-specific hyperimmune plasma are not completely effective and carry some risks for foals12,13,14,15. Chemoprophylaxis with macrolides or other classes of antimicrobials is not recommended because of emerging resistance of R. equi (and other bacteria) to these drugs16. Thus, control of R. equi pneumonia in foals relies on antimicrobial treatment of foals affected with either clinical or subclinical pneumonia17.
Macrolides have been the standard antibiotic used for decades to treat R. equi16. Resistance to macrolides in R. equi is an emerging problem in equine medicine that has been linked to the practice of widespread antimicrobial treatment of foals with subclinical pneumonia at farms with endemic R. equi17,18,19,20,21,22. Because effective alternative antimicrobials for treating foals with R. equi infections are limited18,23,24,25,26, there is an urgent need for non-antibiotic approaches such as host-directed therapy (HDT) to fight these pathogens. Immunotherapy targeting innate immune responses in neonates is an important strategy for HDT because the immune system of foals is immature4,5,6,7,8,9 and infection with R. equi appears to occur early in life2 when foals are known to be more susceptible to this bacterium3.
Toll-like receptors (TLRs) are among the principal signaling receptors for initiating innate immunity, resulting in various effects including cytokine production27. The intracellular TLR9 recognizes bacterial unmethylated cytosine-phosphate-guanine oligodeoxynucleotides (CpG-ODNs)28. We have previously demonstrated that a B class CpG-ODN induced expression of pro-inflammatory cytokines by immune cells of foals both ex vivo and in vivo4,29,30,31, including interferon-gamma (IFN-γ). Evidence exists that younger foals have lower levels of IFN-γ at birth than older foals7,8, and that stimulation of phagocytes with IFN-γ promotes killing of intracellular bacteria such as R. equi32,33 and Mycobacterium tuberculosis (Mtb)34. The TLR2 recognizes lipoteichoic acid and peptidoglycan of Gram + bacteria. TLR2−/− mice are more susceptible to both R. equi infection35 and Mtb36 indicating that activation of the TLR2 signaling pathway can be important in defense against intracellular bacteria. PUL-042 (Pulmotect, Inc., Houston, TX) is an inhaled product that combines two synthetic TLR agonists, Pam2CSK4 (ligand of TLR2/6) and a class C CpG-ODN M362 (ligand of TLR9). This compound drug has been tested in many species against different viral, bacterial, and fungal pathogens and has shown promising results of increasing host survival and reducing pathogen burden37,38,39,40,41,42.
The objective of our research was to test whether nebulization with PUL-042 in foals would improve innate immune responses and reduce the severity and duration of pneumonia due to intrabronchial challenge with R. equi of foals at 28 days of age. We demonstrate that: (1) a combination of TLR2/6 and TLR9 agonists (PUL-042) administered via nebulization at various doses and on multiple occasions is safe for neonatal foals; (2) aerosol treatment of neonatal foals with PUL-042 increased interleukin (IL)-6 and IFN-γ concentration in the supernatant of alveolar macrophages (AMs) upon ex vivo infection; (3) nebulization of PUL-042 in foals challenged intrabronchially with live virulent R. equi reduced the duration of fever, clinical pneumonia, and cough, as well as reduced the size of pulmonary lesions compared to foals that were not nebulized. Our results indicate that nebulization with PUL-042 enhanced functional responses by AMs and reduced the severity of clinical pneumonia compared to non-nebulized foals.
Results
Nebulization with PUL-042 is safe for newborn foals
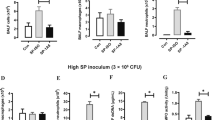
Forty-three foals (36 foals from ex vivo study, seven from in vivo challenge study; Fig. 1A,B) were nebulized multiple times with PUL-042 in this study, and none had any detectable adverse reaction to the nebulization such as increase in respiratory effort and rate, lacrimation, cough, nasal discharge, etc. The percentage of macrophages significantly increased (Fig. 2A) and the percentage of neutrophils decreased (Fig. 2B) with age in BAL cells in all foals, irrespective of treatment. The percentage of lymphocytes did not change with age or treatment group (Fig. 2C). There was no indication of systemic inflammation attributable to nebulization with PUL-042. Serum amyloid A (Fig. 2D), IL-6 (Fig. 3A) and TNF-α (Fig. 3B) decreased with age for all groups independent of their aerosolization treatment. Interleukin-10 concentrations in serum were not altered by nebulization (Fig. 3C). Plasma fibrinogen concentration did not change significantly following treatment (data not shown). These results indicated that nebulization with PUL-042 was safe for newborn foals and did not induce either systemic or local inflammation.
Study design. (A) Ex vivo study; All foals in this study were nebulized with either PUL-042 or placebo, and Rhodococcus equi infections occurred ex vivo in alveolar macrophages (AMs) obtained through broncho-alveolar lavage (BAL). Control group (n = 12) received 2.8% glycerol, the vehicle diluent for PUL-042; PUL-042 2× (n = 12) received 46 µg Pam2 and 68 µg ODN; PUL-042 4× (n = 12) 92.8 µg Pam2 and 136 µg ODN; PUL-042 6× (n = 12) 139.8 µg Pam2 and 204 µg ODN. BAL procedures for collection of fluid and AM were performed on ages 2 (before nebulization) and 22 days (24 h following the last nebulization). Nebulizations started 1 week after the first BAL (approximately day 9 of age), and each foal received 6 nebulizations in a 2-week interval. Infections were performed ex vivo in AMs. (B) In vivo study; PUL-042 6× (n = 7) received 139.8 µg Pam2 and 204 µg ODN; control-nebulized group received 2.8% glycerol (n = 3); and control non-nebulized group (n = 12) did not receive nebulization. Infections were performed in vivo on day 28 of age, and foals were in the study until approximately 12 weeks of age. Nebulized foals received 9 nebulizations: 4 before and 5 after challenge at 28 days of age. Following challenge, twice daily physical examination, and weekly thoracic ultrasounds (TUS) were performed in all foals. At 12 weeks of age or whenever the foal recovers from clinical pneumonia if after 12 weeks of age, a tracheal wash was performed to confirm complete recovery of pneumonia by demonstrating absence of cultured R. equi and evidence of normal cytology on tracheal-wash fluid. Created with BioRender.com.
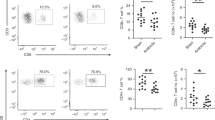
Broncho-alveolar lavage (BAL) fluid differential cytology (A–C) and serum amyloid A (SAA; (D)) of foals either before (Pre-nebulization) or after (Post-nebulization) serial nebulization with PUL-042 at various concentrations: 0 (n = 12), 2× (n = 12), 4× (n = 12), or 6× (n = 12). All foals were nebulized and no foals were infected in vivo with Rhodococcus equi. BALs and blood collection were performed pre- (day 2 after birth) and post-nebulization (day 22, 1 day after the last nebulization). Mean is represented by the symbol (circle for pre-nebulization; diamond for post-nebulization) and 95% confidence interval (whiskers). Percentage of macrophages (A) in BAL increased significantly (P < 0.0001) while percentage of neutrophils (B) decreased (P < 0.0001) after treatment (post) in all groups. The percentage of lymphocytes (C) did not vary significantly. Serum amyloid A (D) significantly decreased post-nebulization in all groups irrespective of treatment (P < 0.0001).
Concentration of pro- and anti-inflammatory cytokines in serum (left panel) or broncho-alveolar lavage (BAL; right panel) fluid of foals either before (Pre-nebulization) or after (Post-nebulization) serial nebulization with PUL-042 at various concentrations: 0 (n = 12), 2× (n = 12), 4× (n = 12), or 6× (n = 12). Cytokines were measured per ml of either serum or BAL fluid (TNF-α = pg/ml; IL-6 = pg/ml; IL-10 = ng/ml). All foals were nebulized and no foals were infected in vivo with Rhodococcus equi. BALs were performed pre (day 2 after birth) and post (day 22, 1 day after the last nebulization). In serum, TNF-⍺ (A) and IL-6 (B) significantly decreased with age, but only IL-6 had 1 group (PUL-042 2X) that was significantly different than PUL-042 0. Serum concentration of IL-10 (C) did not significantly differ among treatment groups or age. Tumor necrosis factor-alpha significantly increased in BAL fluid (D) for all groups, but effects were higher in BAL fluid of PUL-042 treated animals. The concentration of IL-6 (E) and IL-10 (F) in BAL fluid also did not significantly change with either age or treatment. Mean is represented by the symbol (circle for pre-nebulization; diamond for post-nebulization) and 95% confidence interval (whiskers).
Nebulization with PUL-042 influences cytokine production in BAL fluid and AMs infected ex vivo with R. equi
We performed BALs in 48 foals before and after six nebulizations in the second and third week after birth with either 2.8% glycerol or one of three different doses of PUL-042, and collected both BAL cells and fluid (Fig. 1A). Foals nebulized with PUL-042 doses 2× and 4× had increased TNF-α in BAL fluid compared with foals receiving glycerol (Fig. 3D), but no other cytokine (IL-6, IL-10; Fig. 3E,F) concentrations in BAL fluid were altered by treatment. Interleukin-1α and IL-1β were not detected in BAL fluid from foals at either time-point and were not included in the analysis.
Alveolar macrophages from nebulized foals were collected and either kept in media (uninfected, baseline) or infected ex vivo with R. equi (Fig. 1A). The ratio of the concentration of infected/uninfected wells were used because of the high inter-individual variation in baseline production of cytokines. Interleukin-10 and TNF-α in infected cells increased relative to uninfected AMs in all treatment groups including the control foals (Fig. 4A,B), suggesting an age-related increase in response to infection. The AMs from foals nebulized with the two highest doses of PUL-042 that were infected ex vivo with R. equi produced greater IL-6 (Fig. 4C) and IFN-γ compared to cells from foals nebulized with 2.8% glycerol alone (Fig. 4D), indicating PUL-042 resulted in an increased immune response to infection but did not alter responses in uninfected cells. Interleukin-1α and IL-17 were not detected in the supernatant of cultured AMs at either time-point and were not included in the analysis.
Concentration of pro- and anti-inflammatory cytokines in supernatants obtained from cultured alveolar macrophages (AMs) from foals before (age 2 days; Pre-nebulization) and after (age 28 days; Post-nebulization) nebulization with glycerol 2.8% (PUL-042 0; n = 12), and varying doses of PUL-042 (2X, n = 12; 4×, n = 12; 6×, n = 12). All foals were nebulized and no foals were infected in vivo with Rhodococcus equi. Broncho-alveolar lavage was performed pre- (day 2 after birth) and post-nebulization (day 22, 1 day after the last nebulization): AMs were infected with virulent R. equi using a multiplicity of infection of 10 bacteria per macrophage (infected) or kept in media only (uninfected). Supernatant was collected 48 h post-infection for determination of cytokine concentration by ELISA. Data represented as estimates of mean (95% CI); values were analyzed as ratios of R. equi-infected/uninfected cells to account for cytokine production at baseline (control uninfected). Upon ex vivo infection with R. equi, both cytokines TNF-⍺ (A) and IL-10 (B) ratios significantly increased for all groups post-nebulization, while IL-6 ratio significantly increased only after treatment with PUL-042 (C). For INF-γ, this significant increase was only observed for the 2 higher doses of PUL-042, 4× and 6× (D).
Nebulization with PUL-042 does not improve ex vivo killing of R. equi by AMs
Phagocytosis of virulent R. equi by AMs from nebulized foals (Fig. 1A) significantly increased with age in animals of all groups at age 22 days compared with age 2 days (T0; Fig. 5A). Nebulization with PUL-042 did not increase phagocytosis in AMs (Fig. 5A), nor did it improve killing of R. equi intracellularly (ratio of T48/T0; Fig. 5B). Because the balance of pro- and anti-inflammatory cytokines is more important than their individual concentrations, we examined the ratio of stimulated IFN-γ expression (ratio of infected to uninfected cells) and IL-10 expression (ratio of infected to uninfected cells), and found that it was associated with killing of R. equi (Fig. 5C; R value − 0.4740).
Internalization and intracellular killing of Rhodococcus equi by alveolar macrophages (AMs) collected via bronchoalveolar (BAL) lavage pre (day 2 after birth) and post (day 22, 1 day after the last nebulization). All 48 foals were nebulized with either glycerol 2.8% (PUL-042 0; n = 12), or varying doses of PUL-042 (2×, n = 12; 4×, n = 12; or 6×, n = 12). No foals were infected in vivo with R. equi. Alveolar macrophages were infected with 106 virulent R. equi using a multiplicity of infection of 10 bacteria per macrophage (infected) or kept with media only (uninfected control). Cells were then washed and either lysed and diluted immediately (T0) for bacterial determination, or cultured for 48 h on 5% CO2 and then lysed and diluted for CFU counts (T48). There were not significant effects of treatment or age in either phagocytosis ((A); T0) or replication ((B); ratio of T48:T0). (C) CFU ratio (T48:T0) vs IFNg:IL-10. The CFU ratio (lower ratio = greater intracellular killing) was significantly (P = 0.0075) and negatively associated with the ratio of IFN-g:IL-10 expression ratios (infected/uninfected). The log10 CFU ratio decreased by 10(−0.47397) (95% CI, 10(−0.8149639) to 10(−0.132983)). R-value − 0.4740.
Nebulization with PUL-042 reduces severity of pneumonia
After we ensured the safety of nebulization of PUL-042 in 48 foals, and its ability to modulate the immune responses of AMs following ex vivo infection with R. equi through both pro- and anti-inflammatory cytokine production, we examined whether nebulization with PUL-042 before and after intrabronchial infection with live, virulent R. equi decreased the severity of the resultant pneumonia in foals (Fig. 1B). All foals in all groups experimentally infected were confirmed having R. equi clinical pneumonia based on our case-definition: ≥ 2 physical signs, evidence of lung abscessation, and both presence of R. equi and cytologic evidence of suppurative pneumonia with presence of degenerate neutrophils in T-TBA samples. Twenty-two additional foals were randomly assigned to 3 groups: control not nebulized, nebulized before and after infection with 2.8% glycerol (PUL-042 diluent), and nebulized before and after infection with PUL-042. Foals that were nebulized with PUL-042 following the infection had a significantly (P < 0.05) shorter duration of clinical signs of pneumonia, coughing, and fever (Fig. 6A), shorter duration of detection (Fig. 6B), and smaller total sum (Fig. 6C) of TUS lesions when compared to foals that did not receive nebulization, but not when compared to foals nebulized with glycerol only.
Clinical parameters of foals not nebulized (group control no nebulization; n = 12), nebulized with either PUL-042 6× (139.8 µg Pam2:204 µg ODN diluted in 2.8% glycerol; group PUL-042 6×; n = 7) or glycerol 2.8% (group control nebulization; n = 3) before and after infection with 106 virulent R. equi intrabronchially at 28 days of age. (A) Non-nebulized foals showed longer duration of clinical signs of pneumonia, coughing, and fever when compared to foals receiving PUL-042 6×. (B) Nebulization with PUL-042 6× had shorter duration of presence of abscesses in their lungs upon thoracic ultrasound examination when compared to both control groups, although only group control no nebulization was significantly longer. (C) Foals that did not receive any nebulization showed larger sum of ultrasonographic lesions than foals that were nebulized with either PUL-042 6× or glycerol 2.8%. Mean is represented by the symbol (circle for control no-nebulization; square for control nebulization; and triangle for PUL-042 6×) and 95% confidence interval (whiskers).
Discussion
In this study we found that nebulization of foals with PUL-042 after intrabronchial challenge with R. equi reduced the severity of pneumonia in foals. This may be an important finding, because it could be a potential ancillary treatment to antimicrobials for foals with natural R. equi pneumonia. It has been hypothesized that HDTs that modulate TNF-α as an adjunct to canonical chemotherapy shorten therapy duration, reduce pathology, and limit tuberculosis relapse in experimental models by decreasing exacerbated inflammation43. Additionally, as seen in mice with influenza pneumonia and antiviral therapy37, PUL-042 could have a synergistic effect when used in combination with antimicrobials, and it could potentially decrease either the dose of antibiotic or duration of treatment, aiding in the reduction of pressure for development of antimicrobial resistance. In our study, foals were nebulized before clinical pneumonia developed, thus, antimicrobial treatment and nebulization treatments were not contemporaneous. The synergy between concurrent administration of macrolides and PUL-042 in foals with pneumonia merits further evaluation. Surprisingly, we also observed that nebulization procedure with glycerol only (our nebulized control group) was beneficial to reduce severity of disease. Although duration of fever, duration of clinical signs, and duration of lung lesions tended to be higher in the glycerol only group, that difference was not significant. We are not able to explain the reason for this finding. Glycerin compounds are solvents for chemicals in e-cigarettes, and glycerin-based vapors have been shown to increase inflammatory responses in the lungs of mice44,45,46 and to increase cytokine secretion by human AMs and bronchial epithelium47,48,49,50; however, electronic cigarettes contain glycerol in much higher doses (15–100%) than used in our study (2.8%). Glycerol can stimulate pro-inflammatory cytokine secretion by human monocyte-derived macrophages and cultured airway epithelial cells51,52. To the authors’ knowledge, the effects of nebulized glycerol on innate immune responses of equine pulmonary cells have not been investigated, but it is plausible (as suggested by our findings) that nebulization of glycerol solution alone activates innate immune responses, albeit to a lesser extent than the PUL-042. It is possible that glycerol, at low concentrations such as used in this study (2.8%), induces a beneficial inflammatory response. We speculate, however, that the small sample size of the nebulized glycerol vehicle control group precluded us from detecting a significant difference between the PUL-042 and glycerol vehicle. Nevertheless, our results indicate that nebulization with PUL-042 significantly reduced the severity of disease in treated foals.
We measured different cytokines in the supernatant of AMs collected from nebulized foals and infected ex vivo with R. equi. Two important pro-inflammatory cytokines, IL-6 and IFN-γ, were only significantly increased in foals treated with PUL-042. There is evidence that young foals are deficient in IFN-γ, and this deficiency has been theorized to explain why foals are more susceptible to R. equi pneumonia7,8. We have shown previously that a B class CpG-ODN can induce INF-γ in foals, but whether this would translate to a better clinical outcome upon in vivo intrabronchial infection with R. equi, has yet to be determined. Alveolar macrophages are the main cell infected with R. equi; there is, however, evidence that pulmonary epithelial cells have an important role interacting and responding to intracellular bacteria such as R. equi and Mtb53,54,55. There is also evidence that epithelial cells are responsible for inducible resistance conferred by PUL-042 against pneumonia in mice40. Responses to PUL-042 by equine epithelial cells have not been evaluated, but they could be potentially important for lung immunity in vivo. Additionally, the concentration of the pro-inflammatory cytokine TNF-α and anti-inflammatory cytokine IL-10 increased in the fluid supernatant of infected AMs from foals from all groups.
We wanted to determine if nebulization with PUL-042 would increase bactericidal capacity of foal AMs. After collecting BAL cells from foals after six aerosol treatments with PUL-042 and infecting them with live R. equi, we did not observe either an increase in either phagocytosis or in killing of R. equi when compared with foals that received glycerol 2.8% alone. Despite our hypothesis that AMs from foals treated with PUL-042 would kill R. equi better than AMs collected from untreated foals, our negative findings were not unexpected. There is evidence that treating macrophages without the presence of epithelial cells is not sufficient to increase killing of R. equi42, and these cells were not present in our culture system where AMs were infected. Moreover, clearance of R. equi can be mediated by other innate immune cells including neutrophils11,56,57. We conclude that while nebulization with PUL-042 did not directly appear to impact killing of R. equi by AMs, it modulated immune responses to favor improved functional responses that in the pulmonary environment might enhance the capability of pulmonary cells to kill intracellular pathogens.
Because we observed effects of PUL-042 on cytokine production, and these were directly related to bacterial killing33,34,35, we wanted to determine next if cytokine production and bacterial killing were correlated. The CFU ratio (T48/T0; representing bacterial replication) was indeed associated with the ratio of IFN-γ divided by IL-10 in response to infection. The lower the CFU ratio (meaning greater bacterial killing), the higher was the cytokine ratio, suggesting that a balance between pro- and anti-inflammatory cytokines is necessary for intracellular killing of pathogens.
We also showed in this study that nebulization of PUL-042 in foals resulted in no adverse effects such as lacrimation, increased respiratory effort, or nasal discharge in any of the 43 foals nebulized multiple times with PUL-042. We also did not observe any signs of systemic inflammatory responses induced by PUL-042, because concentrations of pro- or anti-inflammatory cytokines or SAA did not increase in serum of foals after nebulization with any nebulization treatment. We found that foals had significantly higher concentrations of SAA at age 2 days than age 28 days, regardless of the treatment group. Serum amyloid A is an acute-phase protein that rapidly increases with pneumonia or when there is an inflammatory stimulus58. To our knowledge, there is no report evaluating in foals either the age-related change in SAA during the first month after birth, nor following nebulization. Possible explanations for our findings are that 2-day-old foals have higher SAA after ingesting colostrum, or that nebulization procedure per se induced inflammation. There is no evidence that nebulization increases SAA, and because we do not have age-matched control foals that were not nebulized in the ex vivo study, we cannot ascertain what caused the age-related reduction in SAA.
We observed a significant decrease in the % of neutrophils and increase in the % of macrophages in BAL fluid from nebulized foals, irrespective of treatment, while the % of lymphocytes remained unchanged. Hostetter et al. (2017) also observed that the % of neutrophils decreased and the % macrophages increased in the first month when compared to 1-week-old foals, but the differences were not significant59. In our experience, in the first 3 days after birth the % of neutrophils are higher than in 1 week-old-foals, which would explain the discrepancy between the two studies.
An increase in the % of neutrophils in BAL fluid would have indicated a transient local inflammation following nebulization, because neutrophil infiltration in the lungs is considered a marker for inflammation. In the case of infection with Streptococcus pneumoniae (Spn) in mice, however, protection against lethal Spn pneumonia conferred by a nebulized Spn lysate was independent of neutrophil recruitment to the lungs60. We do not have evidence in our study that lung inflammation occurred following nebulization, other than an increased in TNF-α in BAL fluid of foals receiving PUL-042 2× and 4× but not 6×. We consider this to be a positive feature of the treatment in newborn foals because exacerbated inflammation can worsen the severity of pneumonia. All other cytokines remained unchanged in the BAL fluid of nebulized foals.
We recognize that additional studies are necessary to optimize the timing and dose for potentially stronger effects of PUL-042 as well as to overcome anatomical challenges to nebulization of horses, such as their longer tracheas, obligate nasal breathing, etc. Nevertheless, our findings that PUL-042 stimulates innate immunity of foals are promising considering the naivety/immaturity of their immune system in the first month after birth4,5,7,8,31. It is important to note that R. equi experimental infections (including the challenge model used in our study) usually induce a more severe pneumonia than observed in natural infection, likely due to the higher number of infecting organisms used in experimental challenges. Thus, it is possible that nebulization with PUL-042 might be more effective against natural infection with lower doses. Nevertheless, our results show potential for improving neonatal immune responses against bacterial respiratory pathogens, and reducing severity of clinical pneumonia following intrabronchial challenge with R. equi.
Material and methods
Ethics statement
All methods were performed in accordance with the relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines. All procedures for this study were reviewed and approved by the Texas A&M University Institutional Animal Care and Use Committee (IACUC), Animal Use Protocol (AUP) # 2014-0358 CA, 2018-0010, and 2018-0354, and the UGA IACUC AUP# A2019 02-008-YI-AO. The foals used in this study were university-owned, and permission for their use was provided in compliance with the procedures of the referent institution’s IACUC. None of the 70 foals died or were euthanized as a result of this study.
Experimental design
The design of our project is summarized in a schematic (Fig. 1A,B). We first evaluated the safety and compared the ex vivo effects of three nebulization doses in 48 foals (Fig. 1A). We then conducted a randomized, controlled experiment of the selected dose of PUL-042 to reduce the severity of pneumonia in 22 foals following experimental challenge (Fig. 1B). Investigators with the responsibility for clinical diagnosis were blinded to the nebulization group of the foals. A thoracic ultrasonographic examination (TUS) was performed prior to both nebulization and intrabronchial challenge to ensure that study foals did not develop naturally-acquired R. equi pneumonia.
Bacteria preparation
Rhodococcus equi strain 33701+ (ATCC reference virulent VapA+ strain; Rockville, MD) was used for ex vivo infection of AMs61, and R. equi strain EIDL 5–331 (a virulent isolate from a Texas foal that died of R. equi pneumonia) was used for in vivo infection of foals62. One CFU of the respective strain was inoculated into 50 ml of brain–heart infusion broth (BHI; BD Biosciences, La Jolla, CA) and shaken for 24 h at 37 °C, and then sub-cultured in 500 ml of BHI broth and shaken for 24 h at 37 °C. The bacterial suspension was centrifuged at 3400×g (5810R, Eppendorf AG, Hamburg, Germany) for 20 min at 4 °C. The BIH broth supernatant was discarded, the pellet resuspended in 100 ml of PBS, centrifuged, and the PBS supernatant discarded. This procedure was repeated twice. The pellet was then resuspended in PBS, and the concentration of bacteria was determined spectrophotometrically (Genesys 20, Thermo Scientific, Waltham, MA, USA). For preparation of infectious inocula, bacterial suspensions were diluted to reach the desired bacterial concentration. Strains were confirmed to be virulent (VapA positive) by polymerase chain reaction (PCR) in each inoculum before infection63.
Nebulization of foals for ex vivo experiments
Forty-eight Quarter-Horse foals were used to evaluate the safety and the ex vivo effects of nebulization with PUL-042 on AMs (Fig. 1A). All foals were monitored daily by a veterinarian (AIB, NDC, SG), had age-appropriate results of complete blood count (CBC) on day 2 of age, and adequate transfer of passive immunity assessed by a commercially-available semi-quantitative immunoassay for serum concentration of total IgG (SNAP test; IDEXX, Inc., Westbrook, ME, USA). All treatments were administered using a commercially available foal nebulizer (Flexineb Portable Equine Nebulizer System-Foal Size, Nortev LTD, Gallaway Ireland). Each corresponding treatment was diluted with 2.8% glycerol (Sigma-Aldrich, St. Louis, MO) in sterile water and administered to the foals three times per week for two weeks (total of 6 nebulizations), starting on day 9 after birth. This frequency was based on previous studies of administration of PUL-042 in laboratory animals64. Previous preclinical studies projected a therapeutic dose of 23.2 µg Pam2 and 34 µg ODN; the doses selected were 2-, 4-, and 6-fold higher (termed PUL-042 2×, PUL-042 4×, and PUL-042 6× dose, respectively)64,65. Foals in both participating institutions (Texas A&M University and University of Georgia; 24 foals in each) were randomly assigned in four groups (Fig. 1A):
-
(1)
control PUL-042 0 (nebulized with 2.8% glycerol; n = 12);
-
(2)
PUL-042 2X (PUL-042 [46 µg Pam2:68 µg ODN], Pulmotect, Inc.; n = 12);
-
(3)
PUL-042 4X (PUL-042 [92.8 µg Pam2:136 µg ODN], Pulmotect, Inc.; n = 12); and,
-
(4)
PUL-042 6X (PUL-042 [139.8 µg Pam2:204 µg ODN], Pulmotect, Inc.; n = 12).
Nebulization of foals for in vivo infection
For the experimental infection study (Fig. 1B), a different group of foals (n = 22) was used. Ten foals were nebulized multiple times pre- and post-challenge using the same nebulizer described above: seven foals were nebulized with PUL-042 6× (139.8 µg Pam2:204 µg ODN diluted in 2.8% glycerol) and three foals were nebulized with 2.8% glycerol. Twelve foals were not nebulized and served as our control non-nebulized group. Our study was powered with the following assumptions: that PUL-042 would reduce the duration of treatment by 7 days, with a standard deviation of 4 days, power of 80%, significance level of 0.05. Calculations indicated we would need seven foals, respectively, but we included an extra 5 control foals to add power. Post hoc, we added three foals to ensure that effects of nebulization of the vehicle alone. All 22 foals were born in the same year and were housed, managed, and infected similarly and contemporaneously with the other foals.
Broncho-alveolar lavage (BAL)
For the ex vivo study (Fig. 1A), a BAL was performed on foals at ages 2 and 22 days, with an interval of 1 week between the first BAL (at age 2 days) and the first nebulization; the second BAL was performed 1 day after the last nebulization. The BAL procedure and processing of BAL fluid was performed as previously described61,66. Briefly, a BAL catheter (Jorgensen Labs, Colorado) was passaged down the trachea and lodged into a bronchus and the air cuff was inflated. Two 60-ml syringes containing 120 ml of sterile saline solution (0.9% NaCl) were infused through the catheter and immediately aspirated, then the process was repeated 60-ml at a time until a total of 360 ml of saline was infused. A 3-ml aliquot of the BAL fluid containing cells was separated for differential cytology by the respective diagnostic laboratories at each institution. BAL cells were centrifuged at 400 ×g for 10 min, washed once with PBS, and then re-suspended in MEM-α (Lonza, Walkersville, MD) containing 10% heat-inactivated horse serum (Gibco, Gaithersburg, MD), 1% l-glutamine–penicillin–streptomycin solution (PSG; Sigma Aldrich, St. Louis, MO) and amphotericin B (25 μg/ml; United States Pharmacopeia, Rockville, MD). Cells were then counted using a cell counter (CellometerAuto T4, Nexelom Bioscience, Lawrence, MA) using trypan blue (Sigma Aldrich, St. Louis, MO) and diluted to a concentration of 1 × 106 live cells/ml. Serum and BAL fluid were analyzed for pro- and anti-inflammatory cytokine (TNF-α, IL-1 α, IL-1 β, IL-6, and IL-10) concentrations using enzyme-linked immunosorbent assay (ELISA) kits performed according to manufacturer’s instructions (Table 1).
Intracellular R. equi killing assay
Macrophage intracellular killing assays were performed as previously described61,66. One ml of cell suspension was placed in triplicates in wells of two 24-well plates: the first plate was used for CFU determination immediately after 40 min of infection to represent phagocytosis (T0) and the second plate was used for CFU determination after 48 h of incubation to represent bacterial survival or replication (T48). Cells were incubated overnight in supplemented MEM-α at 37 °C with 5% CO2. The next morning, cells were washed with warm PBS and 1 × 106 R. equi (ATCC 33701+ strain, multiplicity of infection [MOI] of 10) in 1 ml of fresh MEM-α containing 10% non-heat-inactivated horse serum without amphotericin B and PSG were added, and the infected cells were incubated for 30 min at 37 °C with 5% CO2. Assay controls consisted of wells with media only. Following infection, cells from T0 plates were lysed with 1 ml/well of distilled water, and the suspension was serially diluted and plated out onto BHI agar plates to determine the concentration of live bacteria. Cells from T48 plates were washed with warm PBS, received 2 ml MEM-α containing 10% heat-inactivated horse serum, amphotericin B, and amikacin (8 μg/ml, United States Pharmacopeia, Rockville, MD), and were incubated for 48 h at 37 °C with 5% CO2. After 48 h, cells were submitted to the same lysing procedure as T0 plates. The mean of triplicate wells was used for data analysis. Supernatant of cultured BAL cells were analyzed for pro- and anti-inflammatory cytokine (IL-1α, IL-6, IL-17, IL-10, IFN-γ, and TNF-α) concentrations using ELISA kits performed according to manufacturer’s instructions (Table 1).
In vivo infection of foals
For transendoscopic infection11,62, foals were sedated using intravenous (IV) injection of xylazine hydrochloride (0.5 mg/kg; Vedco, St. Joseph, MO) and IV butorphanol tartrate (0.02 mg/kg; Zoetis, Florham Park, New Jersey). An aseptically-prepared video-endoscope with outer diameter of 9 mm was inserted via the nares into the trachea and passed to the bifurcation of the main-stem bronchus. A 40-ml suspension of virulent EIDL 5–331 R. equi containing approximately 1 × 106 viable bacteria was administered transendoscopically, with 20 ml infused into the right mainstem bronchus and 20 ml into the left mainstem bronchus via a sterilized silastic tube inserted into the endoscope channel. The silastic tube was flushed twice with 20 ml of air after each 20-ml bacterial infusion. Foals and their mares were housed individually in stalls and separately from other mare and foal pairs for one week following experimental infection. After 1 week, these mare/foal pairs were transferred back to their original pasture.
Clinical monitoring
Clinical monitoring was performed as previously published11,62. Physical examination of foals was performed daily from birth by a veterinarian to observe the presence of any adverse effects of the nebulization and signs of exacerbated inflammation of the lungs. The following parameters were recorded: (1) rectal temperature, heart rate, and respiratory rate; (2) tracheal and thoracic auscultation for the presence of abnormal lung sounds (crackles or wheezes, evaluated for both hemithoraces); (3) presence of coughing or nasal discharge; (4) respiratory effort; and, (5) attitude (active or lethargic, including posture and frequency of suckling). For the ex vivo study using non-infected foals, physical examination was performed once daily and continued until 1 week after the second BAL. For infected foals in the in vivo study, physical examination was performed twice daily following infection to detect clinical signs of pneumonia until 12 weeks of age, or until resolution of pneumonia if the foal remained ill after 12 weeks of age. As previously described11,62, animals were also subjected to weekly TUS to identify evidence of peripheral pulmonary consolidation consistent with R. equi pneumonia. Foals were considered to have clinical pneumonia if they demonstrated ≥ 2 of the following clinical signs: coughing at rest; dull attitude (reluctance to rise, lethargy, increased recumbency); rectal temperature > 39.4 °C; respiratory rate ≥ 60 breaths/min; or, increased respiratory effort (manifested by abdominal lift and nostril flaring) plus ultrasonographic evidence of pulmonary consolidation with a maximal diameter of ≥ 2.0 cm, positive results of culture of R. equi from transendoscopic tracheobronchial aspirate (T-TBA) fluid, and cytologic evidence of septic pneumonia from T-TBA fluid. The T-TBA was performed by washing the tracheobronchial tree with 20 ml of sterile PBS solution delivered through a triple-lumen, double-guarded sterile tubing system (MILA International, Inc. Erlanger, KY, USA), and then aspirating fluid from the tracheobronchial tree through the same catheter system. A T-TBA was performed at the time of onset of clinical signs of pneumonia, and in all foals at the termination of the study at age 12 weeks for all foals (end of study).
The outcomes of the in vivo study were the duration of days meeting the case definition of R. equi pneumonia, the number of days with lesions detected on TUS, and sum of the total maximum diameter (TMD) of ultrasonography lesions over the study period11,62. The TMD was determined by summing the maximum diameters of each lesion recorded in the 4th to the 17th intercostal spaces from each foal at every examination; the sum of the TMDs is an index that incorporated both the duration and severity of lesions. Foals diagnosed with R. equi pneumonia were treated with a combination of clarithromycin (7.5 mg/kg; PO; q 12 h) and rifampin (7.5 mg/kg; PO; q 12 h) until both clinical signs and thoracic ultrasonography lesions had resolved11,62.
Data analysis
ELISA and cytology data were analyzed using mixed-effects modeling with the outcome variable being immune/inflammatory parameters of interest (e.g., the proportion of neutrophils or concentration of cytokines in BAL fluid or serum); treatment groups, times (2 days or 22 days), and their interaction terms were modeled as fixed independent variables; individual foal was included as a random-effect term. The proportional killing capacity of R. equi by AMs also were included as an outcome variable. Model fit was assessed by inspection of diagnostic residual plots. All analyses were performed using R software (version 3.5.1, R Foundation for Statistical Computing, Vienna, Austria), and using a significance level of P < 0.05.
For the clinical data, continuous variables were compared among the 3 groups with regard to post-challenge treatment: (1) no nebulization after; (2) nebulization after with glycerol; and, (3) nebulization after with PUL using a generalized linear model with a Gaussian link. Categorical data were summarized as tables and analyzed using Fisher’s exact tests. Analysis was performed using R software with a significance level of P < 0.05.
References
Takai, S. et al. Correlation of in vitro properties of Rhodococcus (Corynebacterium) equi with virulence for mice. Microbiol. Immunol. 29(12), 1175–1184 (1985).
Horowitz, M. L. et al. Application of Sartwell’s model (lognormal distribution of incubation periods) to age at onset and age at death of foals with Rhodococcus equi pneumonia as evidence of perinatal infection. J. Vet. Intern. Med. 15(3), 171–175 (2001).
Sanz, M. et al. The effect of bacterial dose and foal age at challenge on Rhodococcus equi infection. Vet. Microbiol. 167(3–4), 623–631 (2013).
Liu, T. et al. Basal and stimulus-induced cytokine expression is selectively impaired in peripheral blood mononuclear cells of newborn foals. Vaccine 27(5), 674–683 (2009).
Flaminio, M. J. et al. Characterization of peripheral blood and pulmonary leukocyte function in healthy foals. Vet. Immunol. Immunopathol. 73(3–4), 267–285 (2000).
Flaminio, M. J. et al. The effect of CpG-ODN on antigen presenting cells of the foal. J. Immune Based Ther. Vaccines 5, 1 (2007).
Boyd, N. K. et al. Temporal changes in cytokine expression of foals during the first month of life. Vet. Immunol. Immunopathol. 92(1–2), 75–85 (2003).
Breathnach, C. C. et al. Foals are interferon gamma-deficient at birth. Vet. Immunol. Immunopathol. 112(3–4), 199–209 (2006).
Flaminio, M. J. et al. Foal monocyte-derived dendritic cells become activated upon Rhodococcus equi infection. Clin. Vaccine Immunol. 16(2), 176–183 (2009).
Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 7(5), 379–390 (2007).
Cywes-Bentley, C. et al. Antibody to poly-N-acetyl glucosamine provides protection against intracellular pathogens: Mechanism of action and validation in horse foals challenged with Rhodococcus equi. PLoS Pathog. 14(7), e1007160 (2018).
Giguere, S. et al. Evaluation of a commercially available hyperimmune plasma product for prevention of naturally acquired pneumonia caused by Rhodococcus equi in foals. J. Am. Vet. Med. Assoc. 220(1), 59–63 (2002).
Caston, S. S. et al. Effect of hyperimmune plasma on the severity of pneumonia caused by Rhodococcus equi in experimentally infected foals. Vet. Ther. 7(4), 361–375 (2006).
Hurley, J. R. & Begg, A. P. Failure of hyperimmune plasma to prevent pneumonia caused by Rhodococcus equi in foals. Aust. Vet. J. 72(11), 418–420 (1995).
Sanz, M. G. et al. Administration of commercial Rhodococcus equi specific hyperimmune plasma results in variable amounts of IgG against pathogenic bacteria in foals. Vet. Rec. 175(19), 485 (2014).
Giguere, S. et al. Diagnosis, treatment, control, and prevention of infections caused by Rhodococcus equi in foals. J. Vet. Intern. Med. 25(6), 1209–1220 (2011).
Cohen, N. D. & Giguère, S. Rhodococcus equi foal pneumonia. In Infectious Diseases of the Horse (ed. Mair, H. R.) 235–246 (Equine Veterinary Journal Ltd, Cambridgeshire, 2009).
Burton, A. J. et al. Macrolide- and rifampin-resistant Rhodococcus equi on a horse breeding farm, Kentucky, USA. Emerg. Infect. Dis. 19(2), 282–285 (2013).
Huber, L. et al. Prevalence and risk factors associated with emergence of Rhodococcus equi resistance to macrolides and rifampicin in horse-breeding farms in Kentucky, USA. Vet. Microbiol. 235, 243–247 (2019).
Huber, L. et al. Emergence of resistance to macrolides and rifampin in clinical isolates of Rhodococcus equi from foals in central Kentucky, 1995 to 2017. Antimicrob. Agents Chemother. https://doi.org/10.1128/AAC.01714-18 (2019).
Huber, L. et al. Identification of macrolide- and rifampicin-resistant Rhodococcus equi in environmental samples from equine breeding farms in central Kentucky during 2018. Vet. Microbiol. 232, 74–78 (2019).
Alvarez-Narvaez, S. et al. A common practice of widespread antimicrobial use in horse production promotes multi-drug resistance. Sci. Rep. 10(1), 911 (2020).
Giguere, S. et al. Determination of the prevalence of antimicrobial resistance to macrolide antimicrobials or rifampin in Rhodococcus equi isolates and treatment outcome in foals infected with antimicrobial-resistant isolates of R. equi. J. Am. Vet. Med. Assoc. 237(1), 74–81 (2010).
Jacks, S. S., Giguere, S. & Nguyen, A. In vitro susceptibilities of Rhodococcus equi and other common equine pathogens to azithromycin, clarithromycin, and 20 other antimicrobials. Antimicrob. Agents Chemother. 47(5), 1742–1745 (2003).
Womble, A. et al. Pulmonary disposition of tilmicosin in foals and in vitro activity against Rhodococcus equi and other common equine bacterial pathogens. J. Vet. Pharmacol. Ther. 29(6), 561–568 (2006).
Chaffin, M. K., Cohen, N. D. & Martens, R. J. Chemoprophylactic effects of azithromycin against Rhodococcus equi-induced pneumonia among foals at equine breeding farms with endemic infections. J. Am. Vet. Med. Assoc. 232(7), 1035–1047 (2008).
Ingrid, W. et al. Experimental model of equine alveolar macrophage stimulation with TLR ligands. Vet. Immunol. Immunopathol. 155(1–2), 3037 (2013).
Mak, T. & Saunders, M. E. Innate immunity. In The Immune Response: Basic and Clinical Principles (eds Mak, T. & Saunders, M. E.) 69–92 (Elsevier, London, 2006).
Bordin, A. I. et al. Neutrophil function of neonatal foals is enhanced in vitro by CpG oligodeoxynucleotide stimulation. Vet. Immunol. Immunopathol. 145(1–2), 290–297 (2012).
Cohen, N. D. et al. Intramuscular administration of a synthetic CpG-oligodeoxynucleotide modulates functional 1 responses of neutrophils of neonatal foals. PLoS ONE 9, e109865 (2014).
Liu, M. et al. Activation of foal neutrophils at different ages by CpG oligodeoxynucleotides and Rhodococcus equi. Cytokine 48(3), 280–289 (2009).
Darrah, P. A. et al. Cooperation between reactive oxygen and nitrogen intermediates in killing of Rhodococcus equi by activated macrophages. Infect. Immunol. 68(6), 3587–3593 (2000).
Chan, J. et al. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J. Exp. Med. 175(4), 1111–1122 (1992).
Herbst, S., Schaible, U. E. & Schneider, B. E. Interferon gamma activated macrophages kill mycobacteria by nitric oxide induced apoptosis. PLoS ONE 6(5), e19105 (2011).
Darrah, P. A. et al. Innate immune responses to Rhodococcus equi. J. Immunol. 173(3), 1914–1924 (2004).
Drennan, M. B. et al. Toll-like receptor 2-deficient mice succumb to Mycobacterium tuberculosis infection. Am. J. Pathol. 164(1), 49–57 (2004).
Leiva-Juarez, M. M. et al. Combined aerosolized Toll-like receptor ligands are an effective therapeutic agent against influenza pneumonia when co-administered with oseltamivir. Eur. J. Pharmacol. 818, 191–197 (2018).
Kirkpatrick, C. T. et al. Inducible lung epithelial resistance requires multisource reactive oxygen species generation to protect against viral infections. MBio 9(3), e00696 (2018).
Leiva-Juarez, M. M. et al. Inducible epithelial resistance protects mice against leukemia-associated pneumonia. Blood 128(7), 982–992 (2016).
Cleaver, J. O. et al. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal. Immunol. 7(1), 78–88 (2014).
Tuvim, M. J. et al. Synergistic TLR2/6 and TLR9 activation protects mice against lethal influenza pneumonia. PLoS ONE 7(1), e30596 (2012).
Duggan, J. M. et al. Synergistic interactions of TLR2/6 and TLR9 induce a high level of resistance to lung infection in mice. J. Immunol. 186(10), 5916–5926 (2011).
Roca, F. J. & Ramakrishnan, L. TNF dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 153(3), 521–534 (2013).
Werley, M. S. et al. Toxicological assessment of a prototype e-cigaret device and three flavor formulations: A 90-day inhalation study in rats. Inhal. Toxicol. 28(1), 22–38 (2016).
Hwang, J. H. et al. Electronic cigarette inhalation alters innate immunity and airway cytokines while increasing the virulence of colonizing bacteria. J. Mol. Med. (Berl.) 94(6), 667–679 (2016).
Garcia-Arcos, I. et al. Chronic electronic cigarette exposure in mice induces features of COPD in a nicotine-dependent manner. Thorax 71(12), 1119–1129 (2016).
Clapp, P. W. et al. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell Mol. Physiol. 313(2), L278–L292 (2017).
Higham, A. et al. Electronic cigarette exposure triggers neutrophil inflammatory responses. Respir. Res. 17(1), 56 (2016).
Scott, A. et al. Pro-inflammatory effects of e-cigarette vapour condensate on human alveolar macrophages. Thorax 73(12), 1161–1169 (2018).
Reidel, B. et al. E-Cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am. J. Respir. Crit. Care Med. 197(4), 492–501 (2018).
Ween, M. P. et al. Phagocytosis and inflammation: Exploring the effects of the components of E-cigarette vapor on macrophages. Physiol. Rep. 5(16), e13370 (2017).
Escobar, Y. H. et al. In vitro toxicity and chemical characterization of aerosol derived from electronic cigarette humectants using a newly developed exposure system. Chem. Res. Toxicol. 33(7), 1677–1688 (2020).
Reuschl, A. K. et al. Innate activation of human primary epithelial cells broadens the host response to Mycobacterium tuberculosis in the airways. PLoS Pathog. 13(9), e1006577 (2017).
Remuzgo-Martinez, S. et al. Induction of proinflammatory cytokines in human lung epithelial cells during Rhodococcus equi infection. J. Med. Microbiol. 62(Pt 8), 1144–1152 (2013).
Ramos-Vivas, J. et al. Rhodococcus equi human clinical isolates enter and survive within human alveolar epithelial cells. Microbes Infect. 13(5), 438–446 (2011).
Folmar, C. N. et al. In vitro evaluation of complement deposition and opsonophagocytic killing of Rhodococcus equi mediated by poly-N-acetyl glucosamine hyperimmune plasma compared to commercial plasma products. J. Vet. Intern. Med. 33(3), 1493–1499 (2019).
Rocha, J. N. et al. PNAG-specific equine IgG1 mediates significantly greater opsonization and killing of Prescottella equi (formerly Rhodococcus equi) than does IgG4/7. Vaccine 37(9), 1142–1150 (2019).
Gabay, C. & Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 340(6), 448–454 (1999).
Hostetter, S. J. et al. Age-related variation in the cellular composition of equine bronchoalveolar lavage fluid. Vet. Clin. Pathol. 46(2), 344–353 (2017).
Clement, C. G. et al. Stimulation of lung innate immunity protects against lethal pneumococcal pneumonia in mice. Am. J. Respir. Crit. Care Med. 177(12), 1322–1330 (2008).
Berghaus, L. J., Giguere, S. & Sturgill, T. L. Effects of age and macrophage lineage on intracellular survival and cytokine induction after infection with Rhodococcus equi. Vet. Immunol. Immunopathol. 160(1–2), 41–50 (2014).
Rocha, J. N. et al. Oral administration of electron-beam inactivated Rhodococcus equi failed to protect foals against intrabronchial infection with live, virulent R. equi. PLoS ONE 11(2), e0148111 (2016).
Halbert, N. D. et al. Evaluation of a multiplex polymerase chain reaction assay for simultaneous detection of Rhodococcus equi and the vapA gene. Am. J. Vet. Res. 66(8), 1380–1385 (2005).
Alfaro, V. Y. et al. Safety, tolerability, and biomarkers of the treatment of mice with aerosolized Toll-like receptor ligands. Front. Pharmacol. 5, 8 (2014).
Evans, S. E. et al. Inhaled innate immune ligands to prevent pneumonia. Br. J. Pharmacol. 163(1), 195–206 (2011).
Berghaus, L. J. et al. Effects of priming with cytokines on intracellular survival and replication of Rhodococcus equi in equine macrophages. Cytokine 102, 7–11 (2018).
Acknowledgements
The authors would like to thank Grayson Jockey-Club Research Foundation, Morris Animal Foundation, and Link Equine Research Endowment, Texas A&M University for financial support. We would also like to thank Ms. Abigail Malthaner, Mr. Alec Lucas, Ms. Alexis Gooch, Dr. Amanda Hanafi, Ms. Angelica Allegro, Ms. Barrett Barksdale, Dr. Bibiana Petri da Silveira, Mr. Brennen Tobias, Mr. Cory Mabry, Mr. Garrett Wehmeyer, Ms. Loralei Branch, Ms. Madison-Brooke Roberds, Ms. Shelby Zbranek, Ms. Stephanie Grissom, Ms. Sophia Cortez, and Mrs. Susanne Kahn for technical assistance.
Funding
The project was funded by the Grayson Jockey Club Research Foundation (GJCRF). A.I.B. received support from a Morris Animal Foundation (MAF) Fellowship Award (D15EQ-405). N.D.C. is supported by the Patsy Link Chair in Equine Research. PUL-042 used in this project was donated by Pulmotect, Inc. The results have not been reviewed or endorsed by neither MAF nor GJCRF, and the views expressed do not necessarily reflect the views of the MAF/GJCRF, its officers, directors, affiliates or agents. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.I.B. and N.D.C. were responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, writing (original draft, review & editing). S.G. was responsible for conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision. J.M.B. and L.J.B. assisted with methodology and writing (review & editing). B.S. and R.J. assisted with resources and writing (review & editing). M.H. was responsible for conceptualization, investigation, funding acquisition, and writing (review & editing).
Corresponding author
Ethics declarations
Competing interests
Dr. Hook has received compensation as a member of the scientific advisory board of Pulmotect, Inc. and owns stock options in the company. Drs. Scott and Johnson are employees of Pulmotect, Inc. and own stock options in the company. The other authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bordin, A.I., Cohen, N.D., Giguère, S. et al. Host-directed therapy in foals can enhance functional innate immunity and reduce severity of Rhodococcus equi pneumonia. Sci Rep 11, 2483 (2021). https://doi.org/10.1038/s41598-021-82049-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-021-82049-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.