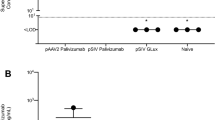
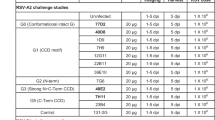
Abstract
Respiratory syncytial virus (RSV) causes acute lower respiratory tract infections, with potential lower respiratory tract infections, which can be particularly problematic in infants and the elderly. There are no approved vaccines for RSV. The current standard of care for high-risk individuals is monthly administration of palivizumab, a humanized murine monoclonal antibody (mAb) targeting the RSV fusion protein. Adeno-associated virus (AAV)-mediated expression of mAbs has previously led to sustained expression of therapeutic concentrations of mAbs in several animal models, representing an alternative to repetitive passive administration. Intramuscular (IM) administration of AAV6.2FF expressing RSV antibodies, palivizumab or hRSV90, resulted in high concentrations of human (h)IgG1 mAbs in the serum and at various mucosal surfaces, while intranasal administration limited hIgG expression to the respiratory tract. IM administration of AAV6.2FF-hRSV90 or AAV6.2FF-palivizumab in a murine model provided sterilizing immunity against challenge with RSV A2. Evidence of maternal passive transfer of vectorized hRSV90 was detected in both murine and ovine models, with circulating mAbs providing sterilizing immunity in mouse progeny. Finally, addition of a “kill switch” comprised of LoxP sites flanking the mAb genes resulted in diminished serum hIgG after AAV-DJ-mediated delivery of Cre recombinase to the same muscle group that was originally transduced with the AAV-mAb vector. The ability of this AAV-mAb system to mediate robust, sustained mAb expression for maternal transfer to progeny in murine and ovine models emphasizes the potential of this platform for use as an alternative prophylactic vaccine for protection against neonatal infections, particularly in high-risk infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data is contained within the article or the supplementary material.
References
Cohen C, Zar HJ. Deaths from RSV in young infants-the hidden community burden. Lancet Glob Health. 2022;10:e169–e170.
Welliver RC, Checchia PA, Bauman JH, Fernandes AW, Mahadevia PJ, Hall CB. Fatality rates in published reports of RSV hospitalizations among high-risk and otherwise healthy children. Curr Med Res Opin. 2010;26:2175–81.
Griffiths C, Drews SJ, Marchant DJ. Respiratory syncytial virus: infection, detection, and new options for prevention and treatment. Clin Microbiol Rev. 2017;30:277–319.
Zhang S, Akmar LZ, Bailey F, Rath BA, Alchikh M, Schweiger B, et al. Cost of respiratory syncytial virus-associated acute lower respiratory infection management in young children at the regional and global level: a systematic review and meta-analysis. J Infect Dis. 2020;222:S680–S687.
Simões EAF, Bont L, Manzoni P, Fauroux B, Paes B, Figueras-Aloy J, et al. Past, present and future approaches to the prevention and treatment of respiratory syncytial virus infection in children. Infect Dis Ther. 2018;7:87–120.
Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–24.
Mousa JJ, Kose N, Matta P, Gilchuk P, Crowe JE. A novel pre-fusion conformation-specific neutralizing epitope on the respiratory syncytial virus fusion protein. Nat Microbiol. 2017;2:16271.
Englund JA. Passive protection against respiratory syncytial virus disease in infants: the role of maternal antibody. Pediatr Infect Dis J. 1994;13:449–53.
Skaricic D, Traube C, De B, Joh J, Boyer J, Crystal RG, et al. Genetic delivery of an anti-RSV antibody to protect against pulmonary infection with RSV. Virology. 2008;378:79–85.
Antepowicz A, Habib O, Kirsebom F, Johansson C, Gill DR, Hyde SC. Lentiviral and AAV-mediated expression of palivizumab offer protection against Respiratory Syncytial Virus infection. Sci Rep. 2021;11:15694.
Guilleman MM, Stevens BAY, Van Lieshout LP, Rghei AD, Pei Y, Santry LA, et al. AAV-mediated delivery of actoxumab and bezlotoxumab results in serum and mucosal antibody concentrations that provide protection from C. difficile toxin challenge. Gene Ther. 2021. https://doi.org/10.1038/s41434-021-00236-y. Online ahead of print.
van Lieshout LP, Domm JM, Rindler TN, Frost KL, Sorensen DL, Medina SJ, et al. A novel triple-mutant AAV6 capsid induces rapid and potent transgene expression in the muscle and respiratory tract of mice. Mol Ther Methods Clin Dev. 2018;9:323–9.
Rghei DA, van Lieshout P, La McLeod MB, Pei Y, Lopes JA, Zielinska N, et al. Safety and tolerability of the adeno-associated virus vector, AAV6.2FF, expressing a monoclonal antibody in murine and ovine animal models. Biomedicines. 2021;9:1186.
Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature. 2012;481:81–4.
Rghei AD, Stevens BAY, Thomas SP, Yates JGE, McLeod BM, Karimi K, et al. Production of adeno-associated virus vectors in cell stacks for preclinical studies in large animal models. J Vis Exp. 2021. https://doi.org/10.3791/62727.
Santry LA, Ingrao JC, Yu DL, de Jong JG, van Lieshout LP, Wood GA, et al. AAV vector distribution in the mouse respiratory tract following four different methods of administration. BMC Biotechnol. 2017;17:43.
Van Hoecke L, Job ER, Saelens X, Roose K. Bronchoalveolar lavage of murine lungs to analyze inflammatory cell infiltration. J Vis Exp. 2017:55398. https://doi.org/10.3791/55398.
Karber G. Breitag zur kollektiven behandlung pharmakologischer reihenversuche. Archiv fur Experimentelle Pathologie und Pharmakologie. 1931;162:480–3.
Zhao B, Yang J, He B, Li X, Yan H, Liu S, et al. A safe and effective mucosal RSV vaccine in mice consisting of RSV phosphoprotein and flagellin variant. Cell Rep. 2021;36:109401.
Barnes MVC, Openshaw PJM, Thwaites RS. Mucosal immune responses to respiratory syncytial virus. Cells. 2022;11:1153.
van Lieshout LP, Soule G, Sorensen D, Frost KL, He S, Tierney K, et al. Intramuscular adeno-associated virus-mediated expression of monoclonal antibodies provides 100% protection against ebola virus infection in mice. J Infect Dis. 2018;217:916–25.
Mousa JJ, Sauer MF, Sevy AM, Finn JA, Bates JT, Alvarado G, et al. Structural basis for nonneutralizing antibody competition at antigenic site II of the respiratory syncytial virus fusion protein. Proc Natl Acad Sci USA. 2016;113:E6849–E6858.
Malinczak CA, Fonseca W, Rasky AJ, Ptaschinski C, Morris S, Ziegler SF, et al. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol. 2019;12:969–79.
Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–15.
Rghei AD, van Lieshout LP, Cao W, He S, Tierney K, Lopes JA, et al. Adeno-associated virus mediated expression of monoclonal antibody MR191 protects mice against Marburg virus and provides long-term expression in sheep. Gene Ther. 2022. https://doi.org/10.1038/s41434-022-00361-2.
Kos CH. Cre/loxP system for generating tissue-specific knockout mouse models. Nutr Rev. 2004;62:243–6.
Loonstra A, Vooijs M, Beverloo HB, Allak BA, van Drunen E, Kanaar R, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci USA. 2001;98:9209–14.
Editorial staff. Cre-recombinase-associated toxicity highlights limitations of genome editing. Dis Model Mech. 2013;6:1299–1300.
Mäe M, Langel U. Cell-penetrating peptides as vectors for peptide, protein and oligonucleotide delivery. Curr Opin Pharmacol. 2006;6:509–14.
Nolden L, Edenhofer F, Peitz M, Brüstle O. Stem cell engineering using transducible Cre recombinase. Methods Mol Med. 2007;140:17–32.
Haupt S, Edenhofer F, Peitz M, Leinhaas A, Brüstle O. Stage-specific conditional mutagenesis in mouse embryonic stem cell-derived neural cells and postmitotic neurons by direct delivery of biologically active Cre recombinase. Stem Cells. 2007;25:181–8.
Nolden L, Edenhofer F, Haupt S, Koch P, Wunderlich FT, Siemen H, et al. Site-specific recombination in human embryonic stem cells induced by cell-permeant Cre recombinase. Nat Methods. 2006;3:461–7.
Zou Z, Sun Z, Li P, Feng T, Wu S. Cre fused with RVG peptide mediates targeted genome editing in mouse brain cells in vivo. Int J Mol Sci. 2016;17:2104.
Sharma A, Wu W, Sung B, Huang J, Tsao T, Li X, et al. Respiratory Syncytial Virus (RSV) Pulmonary Infection in Humanized Mice Induces Human Anti-RSV Immune Responses and Pathology. J Virol. 2016;90:5068–74.
Balasubramani GK, Nowalk MP, Eng H, Zimmerman RK. Estimating the burden of adult hospitalized RSV infection using local and state data - methodology. Hum Vaccin Immunother. 2022;18:1958610.
Resch B, Manzoni P, Lanari M. Severe respiratory syncytial virus (RSV) infection in infants with neuromuscular diseases and immune deficiency syndromes. Paediatr Respir Rev. 2009;10:148–53.
Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, et al. Immunosenescence and its hallmarks: how to oppose aging strategically? A review of potential options for therapeutic intervention. Front Immunol. 2019;10:2247.
Vissers M, Ahout IM, de Jonge MI, Ferwerda G. Mucosal IgG levels correlate better with respiratory syncytial virus load and inflammation than plasma IgG levels. Clin Vaccine Immunol. 2015;23:243–5.
Halfhide CP, Flanagan BF, Brearey SP, Hunt JA, Fonceca AM, McNamara PS, et al. Respiratory syncytial virus binds and undergoes transcription in neutrophils from the blood and airways of infants with severe bronchiolitis. J Infect Dis. 2011;204:451–8.
Yui I, Hoshi A, Shigeta Y, Takami T, Nakayama T. Detection of human respiratory syncytial virus sequences in peripheral blood mononuclear cells. J Med Virol. 2003;70:481–9.
Kang MH, van Lieshout LP, Xu L, Domm JM, Vadivel A, Renesme L, et al. A lung tropic AAV vector improves survival in a mouse model of surfactant B deficiency. Nat Commun. 2020;11:3929.
Allard B, Panariti A, Martin JG. Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection. Front Immunol. 2018;9:1777.
Dekkers G, Bentlage AEH, Stegmann TC, Howie HL, Lissenberg-Thunnissen S, Zimring J, et al. Affinity of human IgG subclasses to mouse Fc gamma receptors. MAbs. 2017;9:767–73.
Taylor G. Animal models of respiratory syncytial virus infection. Vaccine. 2017;35:469–80.
Harker JA, Yamaguchi Y, Culley FJ, Tregoning JS, Openshaw PJ. Delayed sequelae of neonatal respiratory syncytial virus infection are dependent on cells of the innate immune system. J Virol. 2014;88:604–11.
Olivier A, Gallup J, de Macedo MM, Varga SM, Ackermann M. Human respiratory syncytial virus A2 strain replicates and induces innate immune responses by respiratory epithelia of neonatal lambs. Int J Exp Pathol. 2009;90:431–8.
Derscheid RJ, Ackermann MR. Perinatal lamb model of respiratory syncytial virus (RSV) infection. Viruses. 2012;4:2359–78.
Lemaitre J, Naninck T, Delache B, Creppy J, Huber P, Holzapfel M, et al. Non-human primate models of human respiratory infections. Mol Immunol. 2021;135:147–64.
Clemens E, Angeletti D, Holbrook BC, Kanekiyo M, Jorgensen MJ, Graham BS, et al. Influenza-infected newborn and adult monkeys exhibit a strong primary antibody response to hemagglutinin stem. JCI Insight. 2020;5:e135449.
Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simões EAF, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med. 2020;383:426–39.
August A, Glenn GM, Kpamegan E, Hickman SP, Jani D, Lu H, et al. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine. 2017;35:3749–59.
Muňoz FM, Swamy GK, Hickman SP, Agrawal S, Piedra PA, Glenn GM, et al. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J Infect Dis. 2019;220:1802–15.
Backes IM, Byrd BK, Slein MD, Patel CD, Taylor SA, Garland CR, et al. Maternally transferred mAbs protect neonatal mice from HSV-induced mortality and morbidity. J Exp Med. 2022;219:e20220110.
Furukawa S, Kuroda Y, Sugiyama A. A comparison of the histological structure of the placenta in experimental animals. J Toxicol Pathol. 2014;27:11–8.
Soncin F, Natale D, Parast MM. Signaling pathways in mouse and human trophoblast differentiation: a comparative review. Cell Mol Life Sci. 2015;72:1291–302.
Enders AC, Blankenship TN. Comparative placental structure. Adv Drug Deliv Rev. 1999;38:3–15.
Sangild PT. Uptake of colostral immunoglobulins by the compromised newborn farm animal. Acta Vet Scand Suppl. 2003;98:105–22.
Sun J, Shao W, Chen X, Merricks EP, Wimsey L, Abajas YL, et al. An observational study from long-term AAV re-administration in two hemophilia dogs. Mol Ther Methods Clin Dev. 2018;10:257–67.
Grimm D, Lee JS, Wang L, Desai T, Akache B, Storm TA, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82:5887–911.
Mays LE, Wang L, Tenney R, Bell P, Nam HJ, Lin J, et al. Mapping the structural determinants responsible for enhanced T cell activation to the immunogenic adeno-associated virus capsid from isolate rhesus 32.33. J Virol. 2013;87:9473–85.
Chu HY, Tielsch J, Katz J, Magaret AS, Khatry S, LeClerq SC, et al. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol. 2017;95:90–5.
Acknowledgements
We would like to thank all those who were involved in the care of the animals for these studies. This research was funded by grants from the Canadian Institutes of Health Research (CIHR grant # PJ4 179807) and Mitacs Accelerate (IT27042). ADR, JGEY and JAL are all recipients of the OVC scholarship, ADR and JGEY are recipients of the Ontario Graduate Scholarship. ADR was the recipient of a Mitacs Accelerate Studentship.
Author information
Authors and Affiliations
Contributions
Conceptualization, ADR and SKW; methodology, ADR produced the vector and executed the study designs.; ADR and JGEY propagated RSV virus.; JAL, XZ, MMG, YP, LPvL, and LAS assisted with vector cloning, animal work and/or data analysis. Writing-original draft preparation, ADR; writing-review and editing, JGEY, KK, BWB, LS and SKW; supervision, SKW; funding acquisition, SKW. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
LPvL and SKW are inventors on a US patent for the AAV6.2FF capsid. This patent (US20190216949) is licensed to Avamab Pharma Inc., where BT, LPvL and SKW are co-founders and BT serves as an executive. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Ethics approval
This study was conducted according to the guidelines set forth by the Canadian Council on Animal Care (CCAC) and approved by the Animal Care Committee of the University of Guelph (Animal Use Protocol number 4664).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rghei, A.D., Yates, J.G.E., Lopes, J.A. et al. Antibody-based protection against respiratory syncytial virus in mice and their offspring through vectored immunoprophylaxis. Gene Ther (2023). https://doi.org/10.1038/s41434-023-00385-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41434-023-00385-2